 Yorodumi
Yorodumi+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the prasinophyte-specific light-harvesting complex (Lhcp)from Ostreococcus tauri | |||||||||||||||
 Map data Map data | ||||||||||||||||
 Sample Sample |
| |||||||||||||||
 Keywords Keywords | Complex / Electron transport / Light-harvesting / Photosynthesis | |||||||||||||||
| Function / homology |  Function and homology information Function and homology informationphotosynthesis, light harvesting in photosystem I / photosystem I / photosystem II / chloroplast thylakoid membrane / chlorophyll binding / response to light stimulus Similarity search - Function | |||||||||||||||
| Biological species |  Ostreococcus tauri (plant) Ostreococcus tauri (plant) | |||||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.44 Å | |||||||||||||||
 Authors Authors | Shan J / Sheng X / Ishii A / Watanabe A / Song C / Murata K / Minagawa J / Liu Z | |||||||||||||||
| Funding support |  Japan, Japan,  China, 4 items China, 4 items
| |||||||||||||||
 Citation Citation |  Journal: Elife / Year: 2023 Journal: Elife / Year: 2023Title: The photosystem I supercomplex from a primordial green alga harbors three light-harvesting complex trimers. Authors: Asako Ishii / Jianyu Shan / Xin Sheng / Eunchul Kim / Akimasa Watanabe / Makio Yokono / Chiyo Noda / Chihong Song / Kazuyoshi Murata / Zhenfeng Liu / Jun Minagawa /   Abstract: As a ubiquitous picophytoplankton in the ocean and an early-branching green alga, is a model prasinophyte species for studying the functional evolution of the light-harvesting systems in ...As a ubiquitous picophytoplankton in the ocean and an early-branching green alga, is a model prasinophyte species for studying the functional evolution of the light-harvesting systems in photosynthesis. Here, we report the structure and function of the photosystem I (PSI) supercomplex in low light conditions, where it expands its photon-absorbing capacity by assembling with the light-harvesting complexes I (LHCI) and a prasinophyte-specific light-harvesting complex (Lhcp). The architecture of the supercomplex exhibits hybrid features of the plant-type and the green algal-type PSI supercomplexes, consisting of a PSI core, an Lhca1-Lhca4-Lhca2-Lhca3 belt attached on one side and an Lhca5-Lhca6 heterodimer associated on the other side between PsaG and PsaH. Interestingly, nine Lhcp subunits, including one Lhcp1 monomer with a phosphorylated amino-terminal threonine and eight Lhcp2 monomers, oligomerize into three trimers and associate with PSI on the third side between Lhca6 and PsaK. The Lhcp1 phosphorylation and the light-harvesting capacity of PSI were subjected to reversible photoacclimation, suggesting that the formation of PSI-LHCI-Lhcp supercomplex is likely due to a phosphorylation-dependent mechanism induced by changes in light intensity. Notably, this supercomplex did not exhibit far-red peaks in the 77 K fluorescence spectra, which is possibly due to the weak coupling of the chlorophyll 603-609 pair in Lhca1-4. | |||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_34736.map.gz emd_34736.map.gz | 157.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-34736-v30.xml emd-34736-v30.xml emd-34736.xml emd-34736.xml | 17.2 KB 17.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_34736_fsc.xml emd_34736_fsc.xml | 12.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_34736.png emd_34736.png | 52.5 KB | ||
| Filedesc metadata |  emd-34736.cif.gz emd-34736.cif.gz | 6.1 KB | ||
| Others |  emd_34736_half_map_1.map.gz emd_34736_half_map_1.map.gz emd_34736_half_map_2.map.gz emd_34736_half_map_2.map.gz | 172 MB 172 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-34736 http://ftp.pdbj.org/pub/emdb/structures/EMD-34736 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34736 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34736 | HTTPS FTP |
-Validation report
| Summary document |  emd_34736_validation.pdf.gz emd_34736_validation.pdf.gz | 942.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_34736_full_validation.pdf.gz emd_34736_full_validation.pdf.gz | 941.8 KB | Display | |
| Data in XML |  emd_34736_validation.xml.gz emd_34736_validation.xml.gz | 20.4 KB | Display | |
| Data in CIF |  emd_34736_validation.cif.gz emd_34736_validation.cif.gz | 26.1 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34736 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34736 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34736 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34736 | HTTPS FTP |
-Related structure data
| Related structure data |  8hg6MC  7ycaC  8hg3C  8hg5C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_34736.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_34736.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.04 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_34736_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_34736_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Prasinophyte-specific Lhc protein (Lhcp) complex
| Entire | Name: Prasinophyte-specific Lhc protein (Lhcp) complex |
|---|---|
| Components |
|
-Supramolecule #1: Prasinophyte-specific Lhc protein (Lhcp) complex
| Supramolecule | Name: Prasinophyte-specific Lhc protein (Lhcp) complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Ostreococcus tauri (plant) / Strain: OTH95 / Organelle: chloroplast / Location in cell: thylakoid membrane Ostreococcus tauri (plant) / Strain: OTH95 / Organelle: chloroplast / Location in cell: thylakoid membrane |
-Macromolecule #1: Chlorophyll a-b binding protein, chloroplastic
| Macromolecule | Name: Chlorophyll a-b binding protein, chloroplastic / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Ostreococcus tauri (plant) Ostreococcus tauri (plant) |
| Molecular weight | Theoretical: 24.689807 KDa |
| Recombinant expression | Organism:  Ostreococcus tauri (plant) Ostreococcus tauri (plant) |
| Sequence | String: MSALLASSFV SRVAAFKAQK VQNKSVSTTV KADIYPEFGT YPGGGESPII PFGSEKNAER EVIHGRWAML GVTGAWAAEN GTGIPWFTA GTLCTPDDCT AVADKFPGAV APLAPEGSGY PSFWNVLIIE IVLVGAAEAY RTGISDSPFD DGLTVGDVNP G GRFDPLGL ...String: MSALLASSFV SRVAAFKAQK VQNKSVSTTV KADIYPEFGT YPGGGESPII PFGSEKNAER EVIHGRWAML GVTGAWAAEN GTGIPWFTA GTLCTPDDCT AVADKFPGAV APLAPEGSGY PSFWNVLIIE IVLVGAAEAY RTGISDSPFD DGLTVGDVNP G GRFDPLGL AESGDLEELK IKELKHCRLS MFAWLGCIFQ ALATQEGPIA NWQSHVADPV HSNVLTNAAK GFGFY UniProtKB: Chlorophyll a-b binding protein, chloroplastic |
-Macromolecule #2: CHLOROPHYLL A
| Macromolecule | Name: CHLOROPHYLL A / type: ligand / ID: 2 / Number of copies: 24 / Formula: CLA |
|---|---|
| Molecular weight | Theoretical: 893.489 Da |
| Chemical component information |  ChemComp-CLA: |
-Macromolecule #3: CHLOROPHYLL B
| Macromolecule | Name: CHLOROPHYLL B / type: ligand / ID: 3 / Number of copies: 15 / Formula: CHL |
|---|---|
| Molecular weight | Theoretical: 907.472 Da |
| Chemical component information |  ChemComp-CHL: |
-Macromolecule #4: Chlorophyll c2
| Macromolecule | Name: Chlorophyll c2 / type: ligand / ID: 4 / Number of copies: 3 / Formula: KC2 |
|---|---|
| Molecular weight | Theoretical: 608.926 Da |
| Chemical component information |  ChemComp-KC2: |
-Macromolecule #5: (1~{S})-3,5,5-trimethyl-4-[(3~{E},5~{E},7~{E},9~{E},11~{E},13~{E}...
| Macromolecule | Name: (1~{S})-3,5,5-trimethyl-4-[(3~{E},5~{E},7~{E},9~{E},11~{E},13~{E},15~{E},17~{E})-3,7,12,16-tetramethyl-18-[(1~{R},4~{R})-2,6,6-trimethyl-4-oxidanyl-cyclohex-2-en-1-yl]octadeca- ...Name: (1~{S})-3,5,5-trimethyl-4-[(3~{E},5~{E},7~{E},9~{E},11~{E},13~{E},15~{E},17~{E})-3,7,12,16-tetramethyl-18-[(1~{R},4~{R})-2,6,6-trimethyl-4-oxidanyl-cyclohex-2-en-1-yl]octadeca-3,5,7,9,11,13,15,17-octaenyl]cyclohex-3-en-1-ol type: ligand / ID: 5 / Number of copies: 12 / Formula: Q6L |
|---|---|
| Molecular weight | Theoretical: 570.887 Da |
| Chemical component information |  ChemComp-Q6L: |
-Macromolecule #6: (3~{E},5~{E},7~{E},9~{E},11~{E},13~{E},15~{E},17~{E})-1-[(1~{S},4...
| Macromolecule | Name: (3~{E},5~{E},7~{E},9~{E},11~{E},13~{E},15~{E},17~{E})-1-[(1~{S},4~{S})-2,2-dimethyl-6-methylidene-1,4-bis(oxidanyl)cyclohexyl]-3,7,12,16-tetramethyl-18-[(1~{R},4~{R})-2,6,6-trimethyl-4-oxidanyl- ...Name: (3~{E},5~{E},7~{E},9~{E},11~{E},13~{E},15~{E},17~{E})-1-[(1~{S},4~{S})-2,2-dimethyl-6-methylidene-1,4-bis(oxidanyl)cyclohexyl]-3,7,12,16-tetramethyl-18-[(1~{R},4~{R})-2,6,6-trimethyl-4-oxidanyl-cyclohex-2-en-1-yl]octadeca-3,5,7,9,11,13,15,17-octaen-2-one type: ligand / ID: 6 / Number of copies: 5 / Formula: IWJ |
|---|---|
| Molecular weight | Theoretical: 600.87 Da |
| Chemical component information | 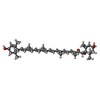 ChemComp-IWJ: |
-Macromolecule #7: (1R,3R)-6-{(3E,5E,7E,9E,11E,13E,15E,17E)-18-[(1S,4R,6R)-4-HYDROXY...
| Macromolecule | Name: (1R,3R)-6-{(3E,5E,7E,9E,11E,13E,15E,17E)-18-[(1S,4R,6R)-4-HYDROXY-2,2,6-TRIMETHYL-7-OXABICYCLO[4.1.0]HEPT-1-YL]-3,7,12,16-TETRAMETHYLOCTADECA-1,3,5,7,9,11,13,15,17-NONAENYLIDENE}-1,5,5-TRIMETHYLCYCLOHEXANE-1,3-DIOL type: ligand / ID: 7 / Number of copies: 1 / Formula: NEX |
|---|---|
| Molecular weight | Theoretical: 600.87 Da |
| Chemical component information | 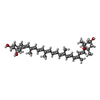 ChemComp-NEX: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 6.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 QUANTUM (4k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Protocol: RIGID BODY FIT |
|---|---|
| Output model |  PDB-8hg6: |
 Movie
Movie Controller
Controller








 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)






































