+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | The cryo-EM structure of hAE2 with bicarbonate | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | chloride and bicarbonate transporter human-AE2 / TRANSPORT PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationnegative regulation of CD8-positive, alpha-beta T cell differentiation / negative regulation of CD8-positive, alpha-beta T cell proliferation / positive regulation of enamel mineralization / Bicarbonate transporters / amelogenesis / monoatomic anion transmembrane transporter activity / chloride:bicarbonate antiporter activity / solute:inorganic anion antiporter activity / digestive tract development / bicarbonate transport ...negative regulation of CD8-positive, alpha-beta T cell differentiation / negative regulation of CD8-positive, alpha-beta T cell proliferation / positive regulation of enamel mineralization / Bicarbonate transporters / amelogenesis / monoatomic anion transmembrane transporter activity / chloride:bicarbonate antiporter activity / solute:inorganic anion antiporter activity / digestive tract development / bicarbonate transport / monoatomic anion transport / regulation of bone resorption / transmembrane transporter activity / osteoclast differentiation / regulation of actin cytoskeleton organization / regulation of intracellular pH / transmembrane transport / spermatogenesis / basolateral plasma membrane / apical plasma membrane / focal adhesion / enzyme binding / membrane / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
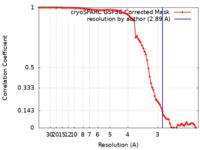
| Method | single particle reconstruction / cryo EM / Resolution: 2.89 Å | |||||||||
 Authors Authors | Zhang Q / Jian L / Yao D / Rao B / Hu K / Xia Y / Cao Y | |||||||||
| Funding support |  China, 2 items China, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2023 Journal: Nat Commun / Year: 2023Title: The structural basis of the pH-homeostasis mediated by the Cl/HCO exchanger, AE2. Authors: Qing Zhang / Liyan Jian / Deqiang Yao / Bing Rao / Ying Xia / Kexin Hu / Shaobai Li / Yafeng Shen / Mi Cao / An Qin / Jie Zhao / Yu Cao /  Abstract: The cell maintains its intracellular pH in a narrow physiological range and disrupting the pH-homeostasis could cause dysfunctional metabolic states. Anion exchanger 2 (AE2) works at high cellular pH ...The cell maintains its intracellular pH in a narrow physiological range and disrupting the pH-homeostasis could cause dysfunctional metabolic states. Anion exchanger 2 (AE2) works at high cellular pH to catalyze the exchange between the intracellular HCO and extracellular Cl, thereby maintaining the pH-homeostasis. Here, we determine the cryo-EM structures of human AE2 in five major operating states and one transitional hybrid state. Among those states, the AE2 shows the inward-facing, outward-facing, and intermediate conformations, as well as the substrate-binding pockets at two sides of the cell membrane. Furthermore, critical structural features were identified showing an interlock mechanism for interactions among the cytoplasmic N-terminal domain and the transmembrane domain and the self-inhibitory effect of the C-terminal loop. The structural and cell-based functional assay collectively demonstrate the dynamic process of the anion exchange across membranes and provide the structural basis for the pH-sensitive pH-rebalancing activity of AE2. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_34290.map.gz emd_34290.map.gz | 59.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-34290-v30.xml emd-34290-v30.xml emd-34290.xml emd-34290.xml | 15 KB 15 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_34290_fsc.xml emd_34290_fsc.xml | 8.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_34290.png emd_34290.png | 76.1 KB | ||
| Filedesc metadata |  emd-34290.cif.gz emd-34290.cif.gz | 6 KB | ||
| Others |  emd_34290_half_map_1.map.gz emd_34290_half_map_1.map.gz emd_34290_half_map_2.map.gz emd_34290_half_map_2.map.gz | 59.2 MB 59.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-34290 http://ftp.pdbj.org/pub/emdb/structures/EMD-34290 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34290 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-34290 | HTTPS FTP |
-Validation report
| Summary document |  emd_34290_validation.pdf.gz emd_34290_validation.pdf.gz | 768.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_34290_full_validation.pdf.gz emd_34290_full_validation.pdf.gz | 768.2 KB | Display | |
| Data in XML |  emd_34290_validation.xml.gz emd_34290_validation.xml.gz | 16.5 KB | Display | |
| Data in CIF |  emd_34290_validation.cif.gz emd_34290_validation.cif.gz | 21.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34290 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34290 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34290 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-34290 | HTTPS FTP |
-Related structure data
| Related structure data |  8gvcMC  8gv8C  8gv9C  8gvaC  8gveC  8gvfC  8gvhC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_34290.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_34290.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.1 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_34290_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_34290_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : hAE2 with bicarbonate
| Entire | Name: hAE2 with bicarbonate |
|---|---|
| Components |
|
-Supramolecule #1: hAE2 with bicarbonate
| Supramolecule | Name: hAE2 with bicarbonate / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 137 KDa |
-Macromolecule #1: Anion exchange protein 2
| Macromolecule | Name: Anion exchange protein 2 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 137.176906 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MSSAPRRPAK GADSFCTPEP ESLGPGTPGF PEQEEDELHR TLGVERFEEI LQEAGSRGGE EPGRSYGEED FEYHRQSSHH IHHPLSTHL PPDARRRKTP QGPGRKPRRR PGASPTGETP TIEEGEEDED EASEAEGARA LTQPSPVSTP SSVQFFLQED D SADRKAER ...String: MSSAPRRPAK GADSFCTPEP ESLGPGTPGF PEQEEDELHR TLGVERFEEI LQEAGSRGGE EPGRSYGEED FEYHRQSSHH IHHPLSTHL PPDARRRKTP QGPGRKPRRR PGASPTGETP TIEEGEEDED EASEAEGARA LTQPSPVSTP SSVQFFLQED D SADRKAER TSPSSPAPLP HQEATPRASK GAQAGTQVEE AEAEAVAVAS GTAGGDDGGA SGRPLPKAQP GHRSYNLQER RR IGSMTGA EQALLPRVPT DEIEAQTLAT ADLDLMKSHR FEDVPGVRRH LVRKNAKGST QSGREGREPG PTPRARPRAP HKP HEVFVE LNELLLDKNQ EPQWRETARW IKFEEDVEEE TERWGKPHVA SLSFRSLLEL RRTLAHGAVL LDLDQQTLPG VAHQ VVEQM VISDQIKAED RANVLRALLL KHSHPSDEKD FSFPRNISAG SLGSLLGHHH GQGAESDPHV TEPLMGGVPE TRLEV ERER ELPPPAPPAG ITRSKSKHEL KLLEKIPENA EATVVLVGCV EFLSRPTMAF VRLREAVELD AVLEVPVPVR FLFLLL GPS SANMDYHEIG RSISTLMSDK QFHEAAYLAD EREDLLTAIN AFLDCSVVLP PSEVQGEELL RSVAHFQRQM LKKREEQ GR LLPTGAGLEP KSAQDKALLQ MVEAAGAAED DPLRRTGRPF GGLIRDVRRR YPHYLSDFRD ALDPQCLAAV IFIYFAAL S PAITFGGLLG EKTQDLIGVS ELIMSTALQG VVFCLLGAQP LLVIGFSGPL LVFEEAFFSF CSSNHLEYLV GRVWIGFWL VFLALLMVAL EGSFLVRFVS RFTQEIFAFL ISLIFIYETF YKLVKIFQEH PLHGCSASNS SEVDGGENMT WAGARPTLGP GNRSLAGQS GQGKPRGQPN TALLSLVLMA GTFFIAFFLR KFKNSRFFPG RIRRVIGDFG VPIAILIMVL VDYSIEDTYT Q KLSVPSGF SVTAPEKRGW VINPLGEKSP FPVWMMVASL LPAILVFILI FMETQITTLI ISKKERMLQK GSGFHLDLLL IV AMGGICA LFGLPWLAAA TVRSVTHANA LTVMSKAVAP GDKPKIQEVK EQRVTGLLVA LLVGLSIVIG DLLRQIPLAV LFG IFLYMG VTSLNGIQFY ERLHLLLMPP KHHPDVTYVK KVRTLRMHLF TALQLLCLAL LWAVMSTAAS LAFPFILILT VPLR MVVLT RIFTDREMKC LDANEAEPVF DEREGVDEYN EMPMPV UniProtKB: Anion exchange protein 2 |
-Macromolecule #2: BICARBONATE ION
| Macromolecule | Name: BICARBONATE ION / type: ligand / ID: 2 / Number of copies: 2 / Formula: BCT |
|---|---|
| Molecular weight | Theoretical: 61.017 Da |
| Chemical component information |  ChemComp-BCT: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 26.0 µm / Nominal defocus min: 10.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)