[English] 日本語
 Yorodumi
Yorodumi- EMDB-33540: Cryo-EM structure of the Mycobacterium smegmatis DNA integrity sc... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the Mycobacterium smegmatis DNA integrity scanning protein (MsDisA). | |||||||||
 Map data Map data | Combined map and sharpened with Bfactor of -50 in Relion | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | second messengers / stress response / c-di-AMP / cryo-EM / CELL CYCLE | |||||||||
| Function / homology |  Function and homology information Function and homology informationdiadenylate cyclase / diadenylate cyclase activity / DNA repair / DNA binding / ATP binding Similarity search - Function | |||||||||
| Biological species |  Mycolicibacterium smegmatis MC2 155 (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria) | |||||||||
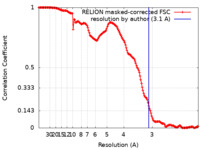
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | |||||||||
 Authors Authors | Gautam S / Vinothkumar KR / Chatterji D | |||||||||
| Funding support |  India, 2 items India, 2 items
| |||||||||
 Citation Citation |  Journal: Protein Sci / Year: 2023 Journal: Protein Sci / Year: 2023Title: Regulatory mechanisms of c-di-AMP synthase from Mycobacterium smegmatis revealed by a structure: Function analysis. Authors: Sudhanshu Gautam / Avisek Mahapa / Lahari Yeramala / Apoorv Gandhi / Sushma Krishnan / Vinothkumar Kutti R / Dipankar Chatterji /  Abstract: Cyclic-di-nucleotide-based secondary messengers regulate various physiological functions, including stress responses in bacteria. Cyclic diadenosine monophosphate (c-di-AMP) has recently emerged as a ...Cyclic-di-nucleotide-based secondary messengers regulate various physiological functions, including stress responses in bacteria. Cyclic diadenosine monophosphate (c-di-AMP) has recently emerged as a crucial second messenger with implications in processes including osmoregulation, antibiotic resistance, biofilm formation, virulence, DNA repair, ion homeostasis, and sporulation, and has potential therapeutic applications. The contrasting activities of the enzymes diadenylate cyclase (DAC) and phosphodiesterase (PDE) determine the equilibrium levels of c-di-AMP. Although c-di-AMP is suspected of playing an essential role in the pathophysiology of bacterial infections and in regulating host-pathogen interactions, the mechanisms of its regulation remain relatively unexplored in mycobacteria. In this report, we biochemically and structurally characterize the c-di-AMP synthase (MsDisA) from Mycobacterium smegmatis. The enzyme activity is regulated by pH and substrate concentration; conditions of significance in the homoeostasis of c-di-AMP levels. Substrate binding stimulates conformational changes in the protein, and pApA and ppApA are synthetic intermediates detectable when enzyme efficiency is low. Unlike the orthologous Bacillus subtilis enzyme, MsDisA does not bind to, and its activity is not influenced in the presence of DNA. Furthermore, we have determined the cryo-EM structure of MsDisA, revealing asymmetry in its structure in contrast to the symmetric crystal structure of Thermotoga maritima DisA. We also demonstrate that the N-terminal minimal region alone is sufficient and essential for oligomerization and catalytic activity. Our data shed light on the regulation of mycobacterial DisA and possible future directions to pursue. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_33540.map.gz emd_33540.map.gz | 301.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-33540-v30.xml emd-33540-v30.xml emd-33540.xml emd-33540.xml | 18.7 KB 18.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_33540_fsc.xml emd_33540_fsc.xml | 15.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_33540.png emd_33540.png | 39.7 KB | ||
| Filedesc metadata |  emd-33540.cif.gz emd-33540.cif.gz | 6.3 KB | ||
| Others |  emd_33540_half_map_1.map.gz emd_33540_half_map_1.map.gz emd_33540_half_map_2.map.gz emd_33540_half_map_2.map.gz | 301.8 MB 301.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33540 http://ftp.pdbj.org/pub/emdb/structures/EMD-33540 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33540 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33540 | HTTPS FTP |
-Validation report
| Summary document |  emd_33540_validation.pdf.gz emd_33540_validation.pdf.gz | 736.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_33540_full_validation.pdf.gz emd_33540_full_validation.pdf.gz | 736.2 KB | Display | |
| Data in XML |  emd_33540_validation.xml.gz emd_33540_validation.xml.gz | 23.4 KB | Display | |
| Data in CIF |  emd_33540_validation.cif.gz emd_33540_validation.cif.gz | 31 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33540 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33540 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33540 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33540 | HTTPS FTP |
-Related structure data
| Related structure data |  7y0dMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_33540.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_33540.map.gz / Format: CCP4 / Size: 325 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Combined map and sharpened with Bfactor of -50 in Relion | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.07 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: One of the half maps from Cryosparc
| File | emd_33540_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | One of the half maps from Cryosparc | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: One of the half maps from Cryosparc
| File | emd_33540_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | One of the half maps from Cryosparc | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Cryo-EM structure of the Mycobacterium smegmatis DNA integrity sc...
| Entire | Name: Cryo-EM structure of the Mycobacterium smegmatis DNA integrity scanning protein (MsDisA). |
|---|---|
| Components |
|
-Supramolecule #1: Cryo-EM structure of the Mycobacterium smegmatis DNA integrity sc...
| Supramolecule | Name: Cryo-EM structure of the Mycobacterium smegmatis DNA integrity scanning protein (MsDisA). type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Mycolicibacterium smegmatis MC2 155 (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria) |
| Molecular weight | Theoretical: 330 KDa |
-Macromolecule #1: DNA integrity scanning protein DisA
| Macromolecule | Name: DNA integrity scanning protein DisA / type: protein_or_peptide / ID: 1 / Number of copies: 8 / Enantiomer: LEVO / EC number: diadenylate cyclase |
|---|---|
| Source (natural) | Organism:  Mycolicibacterium smegmatis MC2 155 (bacteria) Mycolicibacterium smegmatis MC2 155 (bacteria) |
| Molecular weight | Theoretical: 41.844199 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAVKSGARSG RNVVHLARPT LRETLGRLAP GTPLRDGLER ILRGRTGALI VLGYDDSVEA ICDGGFVLDV RYAPTRLREL SKMDGAVVL SSDGSRILRA NVQLVPDPSI PTDESGTRHR SAERTAIQTG YPVISVSHSM SIVTVYVAGE RHVVPDSATI L SRANQTIA ...String: MAVKSGARSG RNVVHLARPT LRETLGRLAP GTPLRDGLER ILRGRTGALI VLGYDDSVEA ICDGGFVLDV RYAPTRLREL SKMDGAVVL SSDGSRILRA NVQLVPDPSI PTDESGTRHR SAERTAIQTG YPVISVSHSM SIVTVYVAGE RHVVPDSATI L SRANQTIA TLERYKGRLD EVSRQLSTAE IEDFVTLRDV MTVVQRLEMV RRISLEIDAD VVELGTDGRQ LKLQLDELVG DN ETARELI VRDYHANPDP PTAAQVAATL EELDSLSDSE LLDFTVLARV FGYPSTAEAQ DSAMSSRGYR AMAAIPRLQF AHV DLLVRS FGSLQNLLAA SADDLQSVDG IGSMWARHIR EGLSLLAEST IADRLAAAAL EHHHHHH UniProtKB: DNA integrity scanning protein DisA |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.1 mg/mL |
|---|---|
| Buffer | pH: 7.5 / Component - Concentration: 75.0 mM / Component - Formula: NaCl / Component - Name: Sodium Chloride Details: Buffer were made freshly with 75mM NaCl and 50mM Tris (pH 7.5) |
| Grid | Model: Quantifoil R2/2 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Atmosphere: AIR |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK IV / Details: blotted for 3.5s. |
| Details | The protein sample was made on a holey carbon grid with an additional layer of thin carbon. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: COUNTING / Digitization - Dimensions - Width: 4096 pixel / Digitization - Dimensions - Height: 4096 pixel / Number grids imaged: 1 / Number real images: 1587 / Average exposure time: 60.0 sec. / Average electron dose: 27.75 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Calibrated magnification: 130841 / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.3000000000000003 µm / Nominal defocus min: 2.1 µm / Nominal magnification: 75000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Details | Model from AlphaFold was used as the starting point (AF-A0R564-F1-model_v2.pdb). |
|---|---|
| Refinement | Space: REAL / Protocol: OTHER / Overall B value: 138.7 |
| Output model |  PDB-7y0d: |
 Movie
Movie Controller
Controller



 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)