+ データを開く
データを開く
- 基本情報
基本情報
| 登録情報 |  | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| タイトル | SARS-CoV-2 Omicron BA.2 Variant RBD complexed with human ACE2 | |||||||||||||||
 マップデータ マップデータ | SARS-CoV-2 Omicron BA.2 Variant RBD complexed with human ACE2 | |||||||||||||||
 試料 試料 |
| |||||||||||||||
 キーワード キーワード | VIRAL PROTEIN-HYDROLASE COMPLEX | |||||||||||||||
| 機能・相同性 |  機能・相同性情報 機能・相同性情報positive regulation of amino acid transport / angiotensin-converting enzyme 2 / positive regulation of L-proline import across plasma membrane / 加水分解酵素; プロテアーゼ; ペプチド結合加水分解酵素; 金属プロテアーゼ / angiotensin-mediated drinking behavior / regulation of systemic arterial blood pressure by renin-angiotensin / positive regulation of gap junction assembly / tryptophan transport / regulation of cardiac conduction / regulation of vasoconstriction ...positive regulation of amino acid transport / angiotensin-converting enzyme 2 / positive regulation of L-proline import across plasma membrane / 加水分解酵素; プロテアーゼ; ペプチド結合加水分解酵素; 金属プロテアーゼ / angiotensin-mediated drinking behavior / regulation of systemic arterial blood pressure by renin-angiotensin / positive regulation of gap junction assembly / tryptophan transport / regulation of cardiac conduction / regulation of vasoconstriction / peptidyl-dipeptidase activity / maternal process involved in female pregnancy / Metabolism of Angiotensinogen to Angiotensins / carboxypeptidase activity / angiotensin maturation / receptor-mediated endocytosis of virus by host cell / Attachment and Entry / metallocarboxypeptidase activity / viral life cycle / positive regulation of cardiac muscle contraction / regulation of cytokine production / blood vessel diameter maintenance / regulation of transmembrane transporter activity / negative regulation of smooth muscle cell proliferation / brush border membrane / negative regulation of ERK1 and ERK2 cascade / metallopeptidase activity / positive regulation of reactive oxygen species metabolic process / endocytic vesicle membrane / regulation of cell population proliferation / virus receptor activity / regulation of inflammatory response / endopeptidase activity / Maturation of spike protein / viral translation / cilium / Translation of Structural Proteins / Virion Assembly and Release / host cell surface / host extracellular space / Potential therapeutics for SARS / symbiont-mediated-mediated suppression of host tetherin activity / Induction of Cell-Cell Fusion / structural constituent of virion / entry receptor-mediated virion attachment to host cell / membrane fusion / host cell endoplasmic reticulum-Golgi intermediate compartment membrane / Attachment and Entry / positive regulation of viral entry into host cell / receptor-mediated virion attachment to host cell / host cell surface receptor binding / symbiont-mediated suppression of host innate immune response / apical plasma membrane / receptor ligand activity / membrane raft / endocytosis involved in viral entry into host cell / endoplasmic reticulum lumen / symbiont entry into host cell / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral envelope / virion attachment to host cell / SARS-CoV-2 activates/modulates innate and adaptive immune responses / host cell plasma membrane / virion membrane / cell surface / extracellular space / extracellular exosome / zinc ion binding / extracellular region / identical protein binding / membrane / plasma membrane 類似検索 - 分子機能 | |||||||||||||||
| 生物種 |   Homo sapiens (ヒト) Homo sapiens (ヒト) | |||||||||||||||
| 手法 | 単粒子再構成法 / クライオ電子顕微鏡法 / 解像度: 3.0 Å | |||||||||||||||
 データ登録者 データ登録者 | Xu Y / Wu C / Liu H / Yin W / Xu HE | |||||||||||||||
| 資金援助 |  中国, 4件 中国, 4件
| |||||||||||||||
 引用 引用 |  ジャーナル: Cell Res / 年: 2022 ジャーナル: Cell Res / 年: 2022タイトル: Structural and biochemical mechanism for increased infectivity and immune evasion of Omicron BA.2 variant compared to BA.1 and their possible mouse origins. 著者: Youwei Xu / Canrong Wu / Xiaodan Cao / Chunyin Gu / Heng Liu / Mengting Jiang / Xiaoxi Wang / Qingning Yuan / Kai Wu / Jia Liu / Deyi Wang / Xianqing He / Xueping Wang / Su-Jun Deng / H Eric Xu / Wanchao Yin /  要旨: The Omicron BA.2 variant has become a dominant infective strain worldwide. Receptor binding studies show that the Omicron BA.2 spike trimer exhibits 11-fold and 2-fold higher potency in binding to ...The Omicron BA.2 variant has become a dominant infective strain worldwide. Receptor binding studies show that the Omicron BA.2 spike trimer exhibits 11-fold and 2-fold higher potency in binding to human ACE2 than the spike trimer from the wildtype (WT) and Omicron BA.1 strains. The structure of the BA.2 spike trimer complexed with human ACE2 reveals that all three receptor-binding domains (RBDs) in the spike trimer are in open conformation, ready for ACE2 binding, thus providing a basis for the increased infectivity of the BA.2 strain. JMB2002, a therapeutic antibody that was shown to efficiently inhibit Omicron BA.1, also shows potent neutralization activities against Omicron BA.2. In addition, both BA.1 and BA.2 spike trimers are able to bind to mouse ACE2 with high potency. In contrast, the WT spike trimer binds well to cat ACE2 but not to mouse ACE2. The structures of both BA.1 and BA.2 spike trimer bound to mouse ACE2 reveal the basis for their high affinity interactions. Together, these results suggest a possible evolution pathway for Omicron BA.1 and BA.2 variants via a human-cat-mouse-human circle, which could have important implications in establishing an effective strategy for combating SARS-CoV-2 viral infections. | |||||||||||||||
| 履歴 |
|
- 構造の表示
構造の表示
| 添付画像 |
|---|
- ダウンロードとリンク
ダウンロードとリンク
-EMDBアーカイブ
| マップデータ |  emd_33341.map.gz emd_33341.map.gz | 352.4 MB |  EMDBマップデータ形式 EMDBマップデータ形式 | |
|---|---|---|---|---|
| ヘッダ (付随情報) |  emd-33341-v30.xml emd-33341-v30.xml emd-33341.xml emd-33341.xml | 22.1 KB 22.1 KB | 表示 表示 |  EMDBヘッダ EMDBヘッダ |
| 画像 |  emd_33341.png emd_33341.png | 26.8 KB | ||
| Filedesc metadata |  emd-33341.cif.gz emd-33341.cif.gz | 7.1 KB | ||
| その他 |  emd_33341_half_map_1.map.gz emd_33341_half_map_1.map.gz emd_33341_half_map_2.map.gz emd_33341_half_map_2.map.gz | 391.2 MB 391.2 MB | ||
| アーカイブディレクトリ |  http://ftp.pdbj.org/pub/emdb/structures/EMD-33341 http://ftp.pdbj.org/pub/emdb/structures/EMD-33341 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33341 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-33341 | HTTPS FTP |
-検証レポート
| 文書・要旨 |  emd_33341_validation.pdf.gz emd_33341_validation.pdf.gz | 741.1 KB | 表示 |  EMDB検証レポート EMDB検証レポート |
|---|---|---|---|---|
| 文書・詳細版 |  emd_33341_full_validation.pdf.gz emd_33341_full_validation.pdf.gz | 740.7 KB | 表示 | |
| XML形式データ |  emd_33341_validation.xml.gz emd_33341_validation.xml.gz | 17.5 KB | 表示 | |
| CIF形式データ |  emd_33341_validation.cif.gz emd_33341_validation.cif.gz | 21 KB | 表示 | |
| アーカイブディレクトリ |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33341 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33341 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33341 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-33341 | HTTPS FTP |
-関連構造データ
| 関連構造データ |  7xo9MC  7xo4C  7xo5C  7xo6C  7xo7C  7xo8C  7xoaC  7xobC  7xocC  7xodC C: 同じ文献を引用 ( M: このマップから作成された原子モデル |
|---|---|
| 類似構造データ | 類似検索 - 機能・相同性  F&H 検索 F&H 検索 |
- リンク
リンク
| EMDBのページ |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| 「今月の分子」の関連する項目 |
- マップ
マップ
| ファイル |  ダウンロード / ファイル: emd_33341.map.gz / 形式: CCP4 / 大きさ: 421.9 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) ダウンロード / ファイル: emd_33341.map.gz / 形式: CCP4 / 大きさ: 421.9 MB / タイプ: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 注釈 | SARS-CoV-2 Omicron BA.2 Variant RBD complexed with human ACE2 | ||||||||||||||||||||||||||||||||||||
| 投影像・断面図 | 画像のコントロール
画像は Spider により作成 | ||||||||||||||||||||||||||||||||||||
| ボクセルのサイズ | X=Y=Z: 0.824 Å | ||||||||||||||||||||||||||||||||||||
| 密度 |
| ||||||||||||||||||||||||||||||||||||
| 対称性 | 空間群: 1 | ||||||||||||||||||||||||||||||||||||
| 詳細 | EMDB XML:
|
-添付データ
-ハーフマップ: SARS-CoV-2 Omicron BA.2 Variant RBD complexed with human...
| ファイル | emd_33341_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
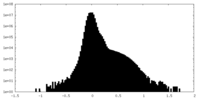
| 注釈 | SARS-CoV-2 Omicron BA.2 Variant RBD complexed with human ACE2 half1 map | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
-ハーフマップ: SARS-CoV-2 Omicron BA.2 Variant RBD complexed with human...
| ファイル | emd_33341_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
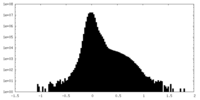
| 注釈 | SARS-CoV-2 Omicron BA.2 Variant RBD complexed with human ACE2 half2 map | ||||||||||||
| 投影像・断面図 |
| ||||||||||||
| 密度ヒストグラム |
- 試料の構成要素
試料の構成要素
-全体 : SARS-CoV-2 Omicron BA.2 Variant RBD complexed with human ACE2
| 全体 | 名称: SARS-CoV-2 Omicron BA.2 Variant RBD complexed with human ACE2 |
|---|---|
| 要素 |
|
-超分子 #1: SARS-CoV-2 Omicron BA.2 Variant RBD complexed with human ACE2
| 超分子 | 名称: SARS-CoV-2 Omicron BA.2 Variant RBD complexed with human ACE2 タイプ: complex / ID: 1 / 親要素: 0 / 含まれる分子: #1-#2 |
|---|---|
| 由来(天然) | 生物種:  |
-超分子 #2: human ACE2
| 超分子 | 名称: human ACE2 / タイプ: complex / ID: 2 / 親要素: 1 / 含まれる分子: #2 |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
-超分子 #3: SARS-CoV-2 Omicron BA.2 Variant RBD
| 超分子 | 名称: SARS-CoV-2 Omicron BA.2 Variant RBD / タイプ: complex / ID: 3 / 親要素: 1 / 含まれる分子: #1 |
|---|
-分子 #1: Spike glycoprotein
| 分子 | 名称: Spike glycoprotein / タイプ: protein_or_peptide / ID: 1 / コピー数: 1 / 光学異性体: LEVO |
|---|---|
| 由来(天然) | 生物種:  |
| 分子量 | 理論値: 141.065406 KDa |
| 組換発現 | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 配列 | 文字列: MFVFLVLLPL VSSQCVNLIT RTQSYTNSFT RGVYYPDKVF RSSVLHSTQD LFLPFFSNVT WFHAIHVSGT NGTKRFDNPV LPFNDGVYF ASTEKSNIIR GWIFGTTLDS KTQSLLIVNN ATNVVIKVCE FQFCNDPFLD VYYHKNNKSW MESEFRVYSS A NNCTFEYV ...文字列: MFVFLVLLPL VSSQCVNLIT RTQSYTNSFT RGVYYPDKVF RSSVLHSTQD LFLPFFSNVT WFHAIHVSGT NGTKRFDNPV LPFNDGVYF ASTEKSNIIR GWIFGTTLDS KTQSLLIVNN ATNVVIKVCE FQFCNDPFLD VYYHKNNKSW MESEFRVYSS A NNCTFEYV SQPFLMDLEG KQGNFKNLRE FVFKNIDGYF KIYSKHTPIN LGRDLPQGFS ALEPLVDLPI GINITRFQTL LA LHRSYLT PGDSSSGWTA GAAAYYVGYL QPRTFLLKYN ENGTITDAVD CALDPLSETK CTLKSFTVEK GIYQTSNFRV QPT ESIVRF PNITNLCPFD EVFNATRFAS VYAWNRKRIS NCVADYSVLY NFAPFFAFKC YGVSPTKLND LCFTNVYADS FVIR GNEVS QIAPGQTGNI ADYNYKLPDD FTGCVIAWNS NKLDSKVGGN YNYLYRLFRK SNLKPFERDI STEIYQAGNK PCNGV AGFN CYFPLRSYGF RPTYGVGHQP YRVVVLSFEL LHAPATVCGP KKSTNLVKNK CVNFNFNGLT GTGVLTESNK KFLPFQ QFG RDIADTTDAV RDPQTLEILD ITPCSFGGVS VITPGTNTSN QVAVLYQGVN CTEVPVAIHA DQLTPTWRVY STGSNVF QT RAGCLIGAEY VNNSYECDIP IGAGICASYQ TQTKSHGSAS SVASQSIIAY TMSLGAENSV AYSNNSIAIP TNFTISVT T EILPVSMTKT SVDCTMYICG DSTECSNLLL QYGSFCTQLK RALTGIAVEQ DKNTQEVFAQ VKQIYKTPPI KYFGGFNFS QILPDPSKPS KRSPIEDLLF NKVTLADAGF IKQYGDCLGD IAARDLICAQ KFNGLTVLPP LLTDEMIAQY TSALLAGTIT SGWTFGAGP ALQIPFPMQM AYRFNGIGVT QNVLYENQKL IANQFNSAIG KIQDSLSSTP SALGKLQDVV NHNAQALNTL V KQLSSKFG AISSVLNDIL SRLDPPEAEV QIDRLITGRL QSLQTYVTQQ LIRAAEIRAS ANLAATKMSE CVLGQSKRVD FC GKGYHLM SFPQSAPHGV VFLHVTYVPA QEKNFTTAPA ICHDGKAHFP REGVFVSNGT HWFVTQRNFY EPQIITTDNT FVS GNCDVV IGIVNNTVYD PLQPELDSFK EELDKYFKNH TSPDVDLGDI SGINASVVNI QKEIDRLNEV AKNLNESLID LQEL GKYEQ YIKWPWYIWL GFIAGLIAIV MVTIMLCCMT SCCSCLKGCC SCGSCCKFDE DDSEPVLKGV KLHYT UniProtKB: Spike glycoprotein |
-分子 #2: Angiotensin-converting enzyme 2
| 分子 | 名称: Angiotensin-converting enzyme 2 / タイプ: protein_or_peptide / ID: 2 / コピー数: 1 / 光学異性体: LEVO / EC番号: angiotensin-converting enzyme 2 |
|---|---|
| 由来(天然) | 生物種:  Homo sapiens (ヒト) Homo sapiens (ヒト) |
| 分子量 | 理論値: 92.556695 KDa |
| 組換発現 | 生物種:  Trichoplusia ni (イラクサキンウワバ) Trichoplusia ni (イラクサキンウワバ) |
| 配列 | 文字列: MSSSSWLLLS LVAVTAAQST IEEQAKTFLD KFNHEAEDLF YQSSLASWNY NTNITEENVQ NMNNAGDKWS AFLKEQSTLA QMYPLQEIQ NLTVKLQLQA LQQNGSSVLS EDKSKRLNTI LNTMSTIYST GKVCNPDNPQ ECLLLEPGLN EIMANSLDYN E RLWAWESW ...文字列: MSSSSWLLLS LVAVTAAQST IEEQAKTFLD KFNHEAEDLF YQSSLASWNY NTNITEENVQ NMNNAGDKWS AFLKEQSTLA QMYPLQEIQ NLTVKLQLQA LQQNGSSVLS EDKSKRLNTI LNTMSTIYST GKVCNPDNPQ ECLLLEPGLN EIMANSLDYN E RLWAWESW RSEVGKQLRP LYEEYVVLKN EMARANHYED YGDYWRGDYE VNGVDGYDYS RGQLIEDVEH TFEEIKPLYE HL HAYVRAK LMNAYPSYIS PIGCLPAHLL GDMWGRFWTN LYSLTVPFGQ KPNIDVTDAM VDQAWDAQRI FKEAEKFFVS VGL PNMTQG FWENSMLTDP GNVQKAVCHP TAWDLGKGDF RILMCTKVTM DDFLTAHHEM GHIQYDMAYA AQPFLLRNGA NEGF HEAVG EIMSLSAATP KHLKSIGLLS PDFQEDNETE INFLLKQALT IVGTLPFTYM LEKWRWMVFK GEIPKDQWMK KWWEM KREI VGVVEPVPHD ETYCDPASLF HVSNDYSFIR YYTRTLYQFQ FQEALCQAAK HEGPLHKCDI SNSTEAGQKL FNMLRL GKS EPWTLALENV VGAKNMNVRP LLNYFEPLFT WLKDQNKNSF VGWSTDWSPY ADQSIKVRIS LKSALGDKAY EWNDNEM YL FRSSVAYAMR QYFLKVKNQM ILFGEEDVRV ANLKPRISFN FFVTAPKNVS DIIPRTEVEK AIRMSRSRIN DAFRLNDN S LEFLGIQPTL GPPNQPPVSI WLIVFGVVMG VIVVGIVILI FTGIRDRKKK NKARSGENPY ASIDISKGEN NPGFQNTDD VQTSF UniProtKB: Angiotensin-converting enzyme 2 |
-分子 #3: CHLORIDE ION
| 分子 | 名称: CHLORIDE ION / タイプ: ligand / ID: 3 / コピー数: 1 / 式: CL |
|---|---|
| 分子量 | 理論値: 35.453 Da |
-分子 #4: ZINC ION
| 分子 | 名称: ZINC ION / タイプ: ligand / ID: 4 / コピー数: 1 / 式: ZN |
|---|---|
| 分子量 | 理論値: 65.409 Da |
-分子 #5: 2-acetamido-2-deoxy-beta-D-glucopyranose
| 分子 | 名称: 2-acetamido-2-deoxy-beta-D-glucopyranose / タイプ: ligand / ID: 5 / コピー数: 3 / 式: NAG |
|---|---|
| 分子量 | 理論値: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-実験情報
-構造解析
| 手法 | クライオ電子顕微鏡法 |
|---|---|
 解析 解析 | 単粒子再構成法 |
| 試料の集合状態 | particle |
- 試料調製
試料調製
| 緩衝液 | pH: 7.4 |
|---|---|
| 凍結 | 凍結剤: ETHANE / チャンバー内湿度: 100 % |
- 電子顕微鏡法
電子顕微鏡法
| 顕微鏡 | FEI TITAN KRIOS |
|---|---|
| 撮影 | フィルム・検出器のモデル: GATAN K3 (6k x 4k) / 平均電子線量: 50.0 e/Å2 |
| 電子線 | 加速電圧: 300 kV / 電子線源:  FIELD EMISSION GUN FIELD EMISSION GUN |
| 電子光学系 | 照射モード: FLOOD BEAM / 撮影モード: BRIGHT FIELD / 最大 デフォーカス(公称値): 2.5 µm / 最小 デフォーカス(公称値): 0.5 µm |
| 試料ステージ | ホルダー冷却材: NITROGEN |
| 実験機器 |  モデル: Titan Krios / 画像提供: FEI Company |
- 画像解析
画像解析
| 初期モデル | モデルのタイプ: PDB ENTRY PDBモデル - PDB ID: |
|---|---|
| 最終 再構成 | 解像度のタイプ: BY AUTHOR / 解像度: 3.0 Å / 解像度の算出法: FSC 0.143 CUT-OFF / 使用した粒子像数: 576163 |
| 初期 角度割当 | タイプ: ANGULAR RECONSTITUTION |
| 最終 角度割当 | タイプ: ANGULAR RECONSTITUTION |
-原子モデル構築 1
| 精密化 | 空間: REAL / プロトコル: RIGID BODY FIT |
|---|---|
| 得られたモデル |  PDB-7xo9: |
 ムービー
ムービー コントローラー
コントローラー


















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)