+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | TRA module of NuA4 | |||||||||
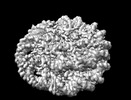
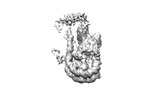
 Map data Map data | TRA1 module | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | NuA4 Tra1 / DNA BINDING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationRHOB GTPase cycle / RHOA GTPase cycle / cellular bud neck contractile ring / piccolo histone acetyltransferase complex / ascospore wall assembly / vacuole inheritance / actin cortical patch / mitotic actomyosin contractile ring contraction / Swr1 complex / SLIK (SAGA-like) complex ...RHOB GTPase cycle / RHOA GTPase cycle / cellular bud neck contractile ring / piccolo histone acetyltransferase complex / ascospore wall assembly / vacuole inheritance / actin cortical patch / mitotic actomyosin contractile ring contraction / Swr1 complex / SLIK (SAGA-like) complex / kinetochore assembly / Ino80 complex / SAGA complex / SWI/SNF complex / establishment of cell polarity / NuA4 histone acetyltransferase complex / actin filament bundle / positive regulation of macroautophagy / protein secretion / chromosome organization / Ub-specific processing proteases / actin filament / Hydrolases; Acting on acid anhydrides; Acting on acid anhydrides to facilitate cellular and subcellular movement / structural constituent of cytoskeleton / endocytosis / transcription corepressor activity / nucleosome / chromatin organization / histone binding / protein-containing complex assembly / hydrolase activity / chromatin remodeling / cell cycle / DNA repair / DNA-templated transcription / chromatin binding / regulation of DNA-templated transcription / chromatin / regulation of transcription by RNA polymerase II / negative regulation of transcription by RNA polymerase II / positive regulation of transcription by RNA polymerase II / ATP binding / identical protein binding / nucleus Similarity search - Function | |||||||||
| Biological species |   | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | |||||||||
 Authors Authors | Chen Z / Qu K | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2022 Journal: Nature / Year: 2022Title: Structure of the NuA4 acetyltransferase complex bound to the nucleosome. Authors: Keke Qu / Kangjing Chen / Hao Wang / Xueming Li / Zhucheng Chen /  Abstract: Deoxyribonucleic acid in eukaryotes wraps around the histone octamer to form nucleosomes, the fundamental unit of chromatin. The N termini of histone H4 interact with nearby nucleosomes and play an ...Deoxyribonucleic acid in eukaryotes wraps around the histone octamer to form nucleosomes, the fundamental unit of chromatin. The N termini of histone H4 interact with nearby nucleosomes and play an important role in the formation of high-order chromatin structure and heterochromatin silencing. NuA4 in yeast and its homologue Tip60 complex in mammalian cells are the key enzymes that catalyse H4 acetylation, which in turn regulates chromatin packaging and function in transcription activation and DNA repair. Here we report the cryo-electron microscopy structure of NuA4 from Saccharomyces cerevisiae bound to the nucleosome. NuA4 comprises two major modules: the catalytic histone acetyltransferase (HAT) module and the transcription activator-binding (TRA) module. The nucleosome is mainly bound by the HAT module and is positioned close to a polybasic surface of the TRA module, which is important for the optimal activity of NuA4. The nucleosomal linker DNA carrying the upstream activation sequence is oriented towards the conserved, transcription activator-binding surface of the Tra1 subunit, which suggests a potential mechanism of NuA4 to act as a transcription co-activator. The HAT module recognizes the disk face of the nucleosome through the H2A-H2B acidic patch and nucleosomal DNA, projecting the catalytic pocket of Esa1 to the N-terminal tail of H4 and supporting its function in selective acetylation of H4. Together, our findings illustrate how NuA4 is assembled and provide mechanistic insights into nucleosome recognition and transcription co-activation by a HAT. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_32149.map.gz emd_32149.map.gz | 13.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-32149-v30.xml emd-32149-v30.xml emd-32149.xml emd-32149.xml | 21.7 KB 21.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_32149_fsc.xml emd_32149_fsc.xml | 13.3 KB | Display |  FSC data file FSC data file |
| Images |  emd_32149.png emd_32149.png | 52.8 KB | ||
| Filedesc metadata |  emd-32149.cif.gz emd-32149.cif.gz | 9.8 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-32149 http://ftp.pdbj.org/pub/emdb/structures/EMD-32149 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32149 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-32149 | HTTPS FTP |
-Validation report
| Summary document |  emd_32149_validation.pdf.gz emd_32149_validation.pdf.gz | 401.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_32149_full_validation.pdf.gz emd_32149_full_validation.pdf.gz | 400.6 KB | Display | |
| Data in XML |  emd_32149_validation.xml.gz emd_32149_validation.xml.gz | 13.5 KB | Display | |
| Data in CIF |  emd_32149_validation.cif.gz emd_32149_validation.cif.gz | 18.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32149 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32149 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32149 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-32149 | HTTPS FTP |
-Related structure data
| Related structure data |  7vvyMC  7vvuC  7vvzC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_32149.map.gz / Format: CCP4 / Size: 202.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_32149.map.gz / Format: CCP4 / Size: 202.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | TRA1 module | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1.0825 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : TRA1 module of NuA4
| Entire | Name: TRA1 module of NuA4 |
|---|---|
| Components |
|
-Supramolecule #1: TRA1 module of NuA4
| Supramolecule | Name: TRA1 module of NuA4 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#6 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Chromatin modification-related protein EAF1
| Macromolecule | Name: Chromatin modification-related protein EAF1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 133.949953 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSSRPSSAVP NSASLSEDQS SDRSKFPKAD DLIDERDRKL TELYCVSRLN QLLELTDENK LRKEIDAFLK KNDIRRGIRF DEASLPKLL HTAATPITKK KLKDVNLINV PNQRLSDSKM SRELPENSEN VSVKSESHFV PSHDNSIREN MMDSLRPAEK T GGMWNKRP ...String: MSSRPSSAVP NSASLSEDQS SDRSKFPKAD DLIDERDRKL TELYCVSRLN QLLELTDENK LRKEIDAFLK KNDIRRGIRF DEASLPKLL HTAATPITKK KLKDVNLINV PNQRLSDSKM SRELPENSEN VSVKSESHFV PSHDNSIREN MMDSLRPAEK T GGMWNKRP LESTMGGEEE RHEKRQKMQS QSLESSNNSE MASLPISPRP PVPNALAHYT YYENIEYPPA DPTEVQPAVK FK DPLIKNI MAKEIDTSDH YNENNVDALE TVFLLMNDYI PSKIPQALPL AELKYMSQTL PLINLIPRAH KALTTNIINN ALN EARITV VGSRIEELRR LGLWSLRQPK RFIDPWKQHN THQNILLEEA KWMQADFKEG HKYKVAICTA MAQAIKDYWT YGEI CCVKR KTLLPGKENK LSDDGRISEK SGRPSDTSRN DSDISIAGKD DIGIIANVDD ITEKESAAAN DNDENGKNEA GAKSD FDFA DGLLSQEGAH DQIISSIDTK LLLKKPSSSS EVVLIQHEVA ASSALIETEE SKKELAPPFK LSIFVDELNT FEKTLI QDL PLYNGINEER PKKDDSLPFI PISKSVVSLD DNGFYKLLER QLIDEEPSIS QLSKRRGMFY GNRRNHYLRP PAVPSLR YL QNRTPTIWLS EDDQELVKNI NTYGYNWELI SAHMTHRLTY SYLSNIERRT PWQCFERFVQ LNERFNFSDL KGPRAHSA Q QWLIEAHKFQ QRQNRRISPL GVNTESIQRG HRRLRWASMF EAIRKCMKKR ENTPRPNPTQ PRKPLDCKNM KVPTPAEMS LLKAQRDEAL RRDIQLRRTV KNRLQQRQQQ SQQAHSSRAQ SPIPSNGKSS SNLARNGQAS APRPNQKQYT EQDIIESYSR KLLEQKPDI GPEMALKAAK NYYRTLREQQ QQLKQHQIQQ QRQQLQEESS HVQQLQQLQP GSQAPPPKSS PSQSSLSNIS N INSAPRIK SPTPQEILQR FQKQRTLQVD WSHPQFEKHH HHHHHHHHHH DYDIPTTASV DGSENLYFQG SPQQNKTAAL AQ HDEAVDN KFNKEQQNAF YEILHLPNLN EEQRNAFIQS LKDDPSQSAN LLAEAKKLND AQAPKVDNKF NKEQQNAFYE ILH LPNLNE EQRNAFIQSL KDDPSQSANL LAEAKKLNDA QAPKVDANSA AL UniProtKB: Chromatin modification-related protein EAF1 |
-Macromolecule #2: Actin-related protein 4
| Macromolecule | Name: Actin-related protein 4 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 54.894684 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSNAALQVYG GDEVSAVVID PGSYTTNIGY SGSDFPQSIL PSVYGKYTAD EGNKKIFSEQ SIGIPRKDYE LKPIIENGLV IDWDTAQEQ WQWALQNELY LNSNSGIPAL LTEPVWNSTE NRKKSLEVLL EGMQFEACYL APTSTCVSFA AGRPNCLVVD I GHDTCSVS ...String: MSNAALQVYG GDEVSAVVID PGSYTTNIGY SGSDFPQSIL PSVYGKYTAD EGNKKIFSEQ SIGIPRKDYE LKPIIENGLV IDWDTAQEQ WQWALQNELY LNSNSGIPAL LTEPVWNSTE NRKKSLEVLL EGMQFEACYL APTSTCVSFA AGRPNCLVVD I GHDTCSVS PIVDGMTLSK STRRNFIAGK FINHLIKKAL EPKEIIPLFA IKQRKPEFIK KTFDYEVDKS LYDYANNRGF FQ ECKETLC HICPTKTLEE TKTELSSTAK RSIESPWNEE IVFDNETRYG FAEELFLPKE DDIPANWPRS NSGVVKTWRN DYV PLKRTK PSGVNKSDKK VTPTEEKEQE AVSKSTSPAA NSADTPNETG KRPLEEEKPP KENNELIGLA DLVYSSIMSS DVDL RATLA HNVVLTGGTS SIPGLSDRLM TELNKILPSL KFRILTTGHT IERQYQSWLG GSILTSLGTF HQLWVGKKEY EEVGV ERLL NDRFR UniProtKB: Actin-related protein 4 |
-Macromolecule #3: Actin
| Macromolecule | Name: Actin / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 41.735547 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MDSEVAALVI DNGSGMCKAG FAGDDAPRAV FPSIVGRPRH QGIMVGMGQK DSYVGDEAQS KRGILTLRYP IEHGIVTNWD DMEKIWHHT FYNELRVAPE EHPVLLTEAP MNPKSNREKM TQIMFETFNV PAFYVSIQAV LSLYSSGRTT GIVLDSGDGV T HVVPIYAG ...String: MDSEVAALVI DNGSGMCKAG FAGDDAPRAV FPSIVGRPRH QGIMVGMGQK DSYVGDEAQS KRGILTLRYP IEHGIVTNWD DMEKIWHHT FYNELRVAPE EHPVLLTEAP MNPKSNREKM TQIMFETFNV PAFYVSIQAV LSLYSSGRTT GIVLDSGDGV T HVVPIYAG FSLPHAILRI DLAGRDLTDY LMKILSERGY SFSTTAEREI VRDIKEKLCY VALDFEQEMQ TAAQSSSIEK SY ELPDGQV ITIGNERFRA PEALFHPSVL GLESAGIDQT TYNSIMKCDV DVRKELYGNI VMSGGTTMFP GIAERMQKEI TAL APSSMK VKIIAPPERK YSVWIGGSIL ASLTTFQQMW ISKQEYDESG PSIVHHKCF UniProtKB: Actin |
-Macromolecule #4: SWR1-complex protein 4
| Macromolecule | Name: SWR1-complex protein 4 / type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 55.297684 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSSSDIFDVL NIKQKSRSPT NGQVSVPSSS AANRPKPQVT GMQRELFNLL GENQPPVVIK SGNNFKEKML STSKPSPWSF VEFKANNSV TLRHWVKGSK ELIGDTPKES PYSKFNQHLS IPSFTKEEYE AFMNENEGTQ KSVESEKNHN ENFTNEKKDE S KNSWSFEE ...String: MSSSDIFDVL NIKQKSRSPT NGQVSVPSSS AANRPKPQVT GMQRELFNLL GENQPPVVIK SGNNFKEKML STSKPSPWSF VEFKANNSV TLRHWVKGSK ELIGDTPKES PYSKFNQHLS IPSFTKEEYE AFMNENEGTQ KSVESEKNHN ENFTNEKKDE S KNSWSFEE IEYLFNLCKK YDLRWFLIFD RYSYNNSRTL EDLKEKFYYT CRNYFKASDP SNPLLSSLNF SAEKEIERKK YL QRLLSRS AAEIAEEEAL VVESKKFEMA AKRTLAERES LLRLLDSPHS DQTITQYLTS QGMSQLYNAL LADKTRKRKH DLN IPENPW MKQQQQFAQH RQLQQLNVKK SEVKENLSPK KTKRQRQEMQ TALKRKSESA YAEQLLKDFN SDERKALGVI THGE KLSPG VYLRSTKLST FKPALQNKIL AILQELSLPS RPVMPSFDVM ERQEELLKKI NTLIDLKKHV DKYEAGMSIT K UniProtKB: SWR1-complex protein 4 |
-Macromolecule #5: Enhancer of polycomb-like protein 1
| Macromolecule | Name: Enhancer of polycomb-like protein 1 / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 96.889867 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MPTPSNAIEI NDGSHKSGRS TRRSGSRSAH DDGLDSFSKG DSGAGASAGS SNSRFRHRKI SVKQHLKIYL PNDLKHLDKD ELQQREVVE IETGVEKNEE KEVHLHRILQ MGSGHTKHKD YIPTPDASMT WNEYDKFYTG SFQETTSYIK FSATVEDCCG T NYNMDERD ...String: MPTPSNAIEI NDGSHKSGRS TRRSGSRSAH DDGLDSFSKG DSGAGASAGS SNSRFRHRKI SVKQHLKIYL PNDLKHLDKD ELQQREVVE IETGVEKNEE KEVHLHRILQ MGSGHTKHKD YIPTPDASMT WNEYDKFYTG SFQETTSYIK FSATVEDCCG T NYNMDERD ETFLNEQVNK GSSDILTEDE FEILCSSFEH AIHERQPFLS MDPESILSFE ELKPTLIKSD MADFNLRNQL NH EINSHKT HFITQFDPVS QMNTRPLIQL IEKFGSKIYD YWRERKIEVN GYEIFPQLKF ERPGEKEEID PYVCFRRREV RHP RKTRRI DILNSQRLRA LHQELKNAKD LALLVAKREN VSLNWINDEL KIFDQRVKIK NLKRSLNISG EDDDLINHKR KRPT IVTVE QREAELRKAE LKRAAAAAAA AKAKNNKRNN QLEDKSSRLT KQQQQQLLQQ QQQQQQNALK TENGKQLANA SSSST SQPI TSHVYVKLPS SKIPDIVLED VDALLNSKEK NARKFVQEKM EKRKIEDADV FFNLTDDPFN PVFDMSLPKN FSTSNV PFA SIASSKFQID RSFYSSHLPE YLKGISDDIR IYDSNGRSRN KDNYNLDTKR IKKTELYDPF QENLEIHSRE YPIKFRK RV GRSNIKYVDR MPNFTTSSTK SACSLMDFVD FDSIEKEQYS REGSNDTDSI NVYDSKYDEF VRLYDKWKYD SPQNEYGI K FSDEPARLNQ ISNDTQVIRF GTMLGTKSYE QLREATIKYR RDYITRLKQK HIQHLQQQQQ QQQQQQQQAQ QQKQKSQNN NSNSSNSLKK LNDSLINSEA KQNSSITQKN SS UniProtKB: Enhancer of polycomb-like protein 1 |
-Macromolecule #6: Transcription-associated protein 1
| Macromolecule | Name: Transcription-associated protein 1 / type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 433.677281 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSLTEQIEQF ASRFRDDDAT LQSRYSTLSE LYDIMELLNS PEDYHFFLQA VIPLLLNQLK EVPISYDAHS PEQKLRNSML DIFNRCLMN QTFQPYAMEV LEFLLSVLPK ENEENGILCM KVLTTLFKSF KSILQDKLDS FIRIIIQIYK NTPNLINQTF Y EAGKAEQG ...String: MSLTEQIEQF ASRFRDDDAT LQSRYSTLSE LYDIMELLNS PEDYHFFLQA VIPLLLNQLK EVPISYDAHS PEQKLRNSML DIFNRCLMN QTFQPYAMEV LEFLLSVLPK ENEENGILCM KVLTTLFKSF KSILQDKLDS FIRIIIQIYK NTPNLINQTF Y EAGKAEQG DLDSPKEPQA DELLDEFSKN DEEKDFPSKQ SSTEPRFENS TSSNGLRSSM FSFKILSECP ITMVTLYSSY KQ LTSTSLP EFTPLIMNLL NIQIKQQQEA REQAESRGEH FTSISTEIIN RPAYCDFILA QIKATSFLAY VFIRGYAPEF LQD YVNFVP DLIIRLLQDC PSELSSARKE LLHATRHILS TNYKKLFLPK LDYLFDERIL IGNGFTMHET LRPLAYSTVA DFIH NIRSE LQLSEIEKTI KIYTGYLLDE SLALTVQIMS AKLLLNLVER ILKLGKENPQ EAPRAKKLLM IIIDSYMNRF KTLNR QYDT IMKYYGRYET HKKEKAEKLK NSIQDNDKES EEFMRKVLEP SDDDHLMPQP KKEDINDSPD VEMTESDKVV KNDVEM FDI KNYAPILLLP TPTNDPIKDA FYLYRTLMSF LKTIIHDLKV FNPPPNEYTV ANPKLWASVS RVFSYEEVIV FKDLFHE CI IGLKFFKDHN EKLSPETTKK HFDISMPSLP VSATKDAREL MDYLAFMFMQ MDNATFNEII EQELPFVYER MLEDSGLL H VAQSFLTSEI TSPNFAGILL RFLKGKLKDL GNVDFNTSNV LIRLFKLSFM SVNLFPNINE VVLLPHLNDL ILNSLKYST TAEEPLVYFY LIRTLFRSIG GGRFENLYRS IKPILQVLLQ SLNQMILTAR LPHERELYVE LCITVPVRLS VLAPYLPFLM KPLVFALQQ YPDLVSQGLR TLELCIDNLT AEYFDPIIEP VIDDVSKALF NLLQPQPFNH AISHNVVRIL GKLGGRNRQF L KPPTDLTE KTELDIDAIA DFKINGMPED VPLSVTPGIQ SALNILQSYK SDIHYRKSAY KYLTCVLLLM TKSSAEFPTN YT ELLKTAV NSIKLERIGI EKNFDLEPTV NKRDYSNQEN LFLRLLESVF YATSIKELKD DAMDLLNNLL DHFCLLQVNT TLL NKRNYN GTFNIDLKNP NFMLDSSLIL DAIPFALSYY IPEVREVGVL AYKRIYEKSC LIYGEELALS HSFIPELAKQ FIHL CYDET YYNKRGGVLG IKVLIDNVKS SSVFLKKYQY NLANGLLFVL KDTQSEAPSA ITDSAEKLLI DLLSITFADV KEEDL GNKV LENTLTDIVC ELSNANPKVR NACQKSLHTI SNLTGIPIVK LMDHSKQFLL SPIFAKPLRA LPFTMQIGNV DAITFC LSL PNTFLTFNEE LFRLLQESIV LADAEDESLS TNIQKTTEYS TSEQLVQLRI ACIKLLAIAL KNEEFATAQQ GNIRIRI LA VFFKTMLKTS PEIINTTYEA LKGSLAENSK LPKELLQNGL KPLLMNLSDH QKLTVPGLDA LSKLLELLIA YFKVEIGR K LLDHLTAWCR VEVLDTLFGQ DLAEQMPTKI IVSIINIFHL LPPQADMFLN DLLLKVMLLE RKLRLQLDSP FRTPLARYL NRFHNPVTEY FKKNMTLRQL VLFMCNIVQR PEAKELAEDF EKELDNFYDF YISNIPKNQV RVVSFFTNMV DLFNTMVITN GDEWLKKKG NMILKLKDML NLTLKTIKEN SFYIDHLQLN QSIAKFQALY LRFTELSERD QNPLLLDFID FSFSNGIKAS Y SLKKFIFH NIIASSNKEK QNNFINDATL FVLSDKCLDA RIFVLKNVIN STLIYEVATS GSLKSYLVED KKPKWLELLH NK IWKNSNA ILAYDVLDHH DLFRFELLQL SAIFIKADPE IIAEIKKDII KFCWNFIKLE DTLIKQSAYL VTSYFISKFD FPI KVVTQV FVALLRSSHV EARYLVKQSL DVLTPVLHER MNAAGTPDTW INWVKRVMVE NSSSQNNILY QFLISHPDLF FNSR DLFIS NIIHHMNKIT FMSNSNSDSH TLAIDLASLI LYWENKTLEI TNVNNTKTDS DGDVVMSDSK SDINPVEADT TAIIV DANN NSPISLHLRE ACTAFLIRYV CASNHRAIET ELGLRAINIL SELISDKHWT NVNVKLVYFE KFLIFQDLDS ENILYY CMN ALDVLYVFFK NKTKEWIMEN LPTIQNLLEK CIKSDHHDVQ EALQKVLQVI MKAIKAQGVS VIIEEESPGK TFIQMLT SV ITQDLQETSS VTAGVTLAWV LFMNFPDNIV PLLTPLMKTF SKLCKDHLSI SQPKDAMALE EARITTKLLE KVLYILSL K VSLLGDSRRP FLSTVALLID HSMDQNFLRK IVNMSRSWIF NTEIFPTVKE KAAILTKMLA FEIRGEPSLS KLFYEIVLK LFDQEHFNNT EITVRMEQPF LVGTRVEDIG IRKRFMTILD NSLERDIKER LYYVIRDQNW EFIADYPWLN QALQLLYGSF NREKELSLK NIYCLSPPSI LQEYLPENAE MVTEVNDLEL SNFVKGHIAS MQGLCRIISS DFIDSLIEIF YQDPKAIHRA W VTLFPQVY KSIPKNEKYG FVRSIITLLS KPYHTRQISS RTNVINMLLD SISKIESLEL PPHLVKYLAI SYNAWYQSIN IL ESIQSNT SIDNTKIIEA NEDALLELYV NLQEEDMFYG LWRRRAKYTE TNIGLSYEQI GLWDKAQQLY EVAQVKARSG ALP YSQSEY ALWEDNWIQC AEKLQHWDVL TELAKHEGFT DLLLECGWRV ADWNSDRDAL EQSVKSVMDV PTPRRQMFKT FLAL QNFAE SRKGDQEVRK LCDEGIQLSL IKWVSLPIRY TPAHKWLLHG FQQYMEFLEA TQIYANLHTT TVQNLDSKAQ EIKRI LQAW RDRLPNTWDD VNMWNDLVTW RQHAFQVINN AYLPLIPALQ QSNSNSNINT HAYRGYHEIA WVINRFAHVA RKHNMP DVC ISQLARIYTL PNIEIQEAFL KLREQAKCHY QNMNELTTGL DVISNTNLVY FGTVQKAEFF TLKGMFLSKL RAYEEAN QA FATAVQIDLN LAKAWAQWGF FNDRRLSEEP NNISFASNAI SCYLQAAGLY KNSKIRELLC RILWLISIDD ASGMLTNA F DSFRGEIPVW YWITFIPQLL TSLSHKEANM VRHILIRIAK SYPQALHFQL RTTKEDFAVI QRQTMAVMGD KPDTNDRNG RRQPWEYLQE LNNILKTAYP LLALSLESLV AQINDRFKST TDEDLFRLIN VLLIDGTLNY NRLPFPRKNP KLPENTEKNL VKFSTTLLA PYIRPKFNAD FIDNKPDYET YIKRLRYWRR RLENKLDRAS KKENLEVLCP HLSNFHHQKF EDIEIPGQYL L NKDNNVHF IKIARFLPTV DFVRGTHSSY RRLMIRGHDG SVHSFAVQYP AVRHSRREER MFQLYRLFNK SLSKNVETRR RS IQFNLPI AIPLSPQVRI MNDSVSFTTL HEIHNEFCKK KGFDPDDIQD FMADKLNAAH DDALPAPDMT ILKVEIFNSI QTM FVPSNV LKDHFTSLFT QFEDFWLFRK QFASQYSSFV FMSYMMMINN RTPHKIHVDK TSGNVFTLEM LPSRFPYERV KPLL KNHDL SLPPDSPIFH NNEPVPFRLT PNIQSLIGDS ALEGIFAVNL FTISRALIEP DNELNTYLAL FIRDEIISWF SNLHR PIIE NPQLREMVQT NVDLIIRKVA QLGHLNSTPT VTTQFILDCI GSAVSPRNLA RTDVNFMPWF UniProtKB: Transcription-associated protein 1 |
-Macromolecule #7: MAGNESIUM ION
| Macromolecule | Name: MAGNESIUM ION / type: ligand / ID: 7 / Number of copies: 2 / Formula: MG |
|---|---|
| Molecular weight | Theoretical: 24.305 Da |
-Macromolecule #8: ADENOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 8 / Number of copies: 2 / Formula: ATP |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.6 |
|---|---|
| Vitrification | Cryogen name: NITROGEN |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.8 µm / Nominal defocus min: 1.3 µm |
 Movie
Movie Controller
Controller