+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-30668 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo_EM structure of delta N-NPC1L1-EZE | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationvitamin transport / cholesterol import / intestinal cholesterol absorption / lipid transporter activity / response to muscle activity / heterocyclic compound binding / cholesterol binding / cholesterol homeostasis / brush border membrane / response to xenobiotic stimulus / apical plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.58 Å | |||||||||
 Authors Authors | Hu M / Sun S | |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2021 Journal: Sci Adv / Year: 2021Title: Structural insights into the mechanism of human NPC1L1-mediated cholesterol uptake. Authors: Miaoqing Hu / Fan Yang / Yawen Huang / Xin You / Desheng Liu / Shan Sun / Sen-Fang Sui /  Abstract: Niemann-Pick C1-like 1 (NPC1L1) protein plays a central role in the intestinal cholesterol absorption and is the target of a drug, ezetimibe, which inhibits NPC1L1 to reduce cholesterol absorption. ...Niemann-Pick C1-like 1 (NPC1L1) protein plays a central role in the intestinal cholesterol absorption and is the target of a drug, ezetimibe, which inhibits NPC1L1 to reduce cholesterol absorption. Here, we present cryo-electron microscopy structures of human NPC1L1 in apo state, cholesterol-enriched state, and ezetimibe-bound state to reveal molecular details of NPC1L1-mediated cholesterol uptake and ezetimibe inhibition. Comparison of these structures reveals that the sterol-sensing domain (SSD) could respond to the cholesterol level alteration by binding different number of cholesterol molecules. Upon increasing cholesterol level, SSD binds more cholesterol molecules, which, in turn, triggers the formation of a stable structural cluster in SSD, while binding of ezetimibe causes the deformation of the SSD and destroys the structural cluster, leading to the inhibition of NPC1L1 function. These results provide insights into mechanisms of NPC1L1 function and ezetimibe action and are of great significance for the development of new cholesterol absorption inhibitors. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_30668.map.gz emd_30668.map.gz | 36.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-30668-v30.xml emd-30668-v30.xml emd-30668.xml emd-30668.xml | 10.7 KB 10.7 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_30668.png emd_30668.png | 110.6 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-30668 http://ftp.pdbj.org/pub/emdb/structures/EMD-30668 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30668 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-30668 | HTTPS FTP |
-Validation report
| Summary document |  emd_30668_validation.pdf.gz emd_30668_validation.pdf.gz | 378.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_30668_full_validation.pdf.gz emd_30668_full_validation.pdf.gz | 377.8 KB | Display | |
| Data in XML |  emd_30668_validation.xml.gz emd_30668_validation.xml.gz | 6.5 KB | Display | |
| Data in CIF |  emd_30668_validation.cif.gz emd_30668_validation.cif.gz | 7.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30668 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30668 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30668 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-30668 | HTTPS FTP |
-Related structure data
| Related structure data |  7dfzMC  7df8C  7dfwC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_30668.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_30668.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.091 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : delta N-hNPC1L1-EZE
| Entire | Name: delta N-hNPC1L1-EZE |
|---|---|
| Components |
|
-Supramolecule #1: delta N-hNPC1L1-EZE
| Supramolecule | Name: delta N-hNPC1L1-EZE / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  Homo sapiens (human) / Recombinant cell: HEK293 Homo sapiens (human) / Recombinant cell: HEK293 |
-Macromolecule #1: NPC1-like intracellular cholesterol transporter 1
| Macromolecule | Name: NPC1-like intracellular cholesterol transporter 1 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 139.612125 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MAEAGLRGWL LWALLLRLAQ SEPYTTIHQP GYCAFYDECG KNPELSGSLM TLSNVSCLSN TPARKITGDH LILLQKICPR LYTGPNTQA CCSAKQLVSL EASLSITKAL LTRCPACSDN FVNLHCHNTC SPNQSLFINV TRVAQLGAGQ LPAVVAYEAF Y QHSFAEQS ...String: MAEAGLRGWL LWALLLRLAQ SEPYTTIHQP GYCAFYDECG KNPELSGSLM TLSNVSCLSN TPARKITGDH LILLQKICPR LYTGPNTQA CCSAKQLVSL EASLSITKAL LTRCPACSDN FVNLHCHNTC SPNQSLFINV TRVAQLGAGQ LPAVVAYEAF Y QHSFAEQS YDSCSRVRVP AAATLAVGTM CGVYGSALCN AQRWLNFQGD TGNGLAPLDI TFHLLEPGQA VGSGIQPLNE GV ARCNESQ GDDVATCSCQ DCAASCPAIA RPQALDSTFY LGQMPGSLVL IIILCSVFAV VTILLVGFRV APARDKSKMV DPK KGTSLS DKLSFSTHTL LGQFFQGWGT WVASWPLTIL VLSVIPVVAL AAGLVFTELT TDPVELWSAP NSQARSEKAF HDQH FGPFF RTNQVILTAP NRSSYRYDSL LLGPKNFSGI LDLDLLLELL ELQERLRHLQ VWSPEAQRNI SLQDICYAPL NPDNT SLYD CCINSLLQYF QNNRTLLLLT ANQTLMGQTS QVDWKDHFLY CANAPLTFKD GTALALSCMA DYGAPVFPFL AIGGYK GKD YSEAEALIMT FSLNNYPAGD PRLAQAKLWE EAFLEEMRAF QRRMAGMFQV TFMAERSLED EINRTTAEDL PIFATSY IV IFLYISLALG SYSSWSRVMV DSKATLGLGG VAVVLGAVMA AMGFFSYLGI RSSLVILQVV PFLVLSVGAD NIFIFVLE Y QRLPRRPGEP REVHIGRALG RVAPSMLLCS LSEAICFFLG ALTPMPAVRT FALTSGLAVI LDFLLQMSAF VALLSLDSK RQEASRLDVC CCVKPQELPP PGQGEGLLLG FFQKAYAPFL LHWITRGVVL LLFLALFGVS LYSMCHISVG LDQELALPKD SYLLDYFLF LNRYFEVGAP VYFVTTLGYN FSSEAGMNAI CSSAGCNNFS FTQKIQYATE FPEQSYLAIP ASSWVDDFID W LTPSSCCR LYISGPNKDK FCPSTVNSLN CLKNCMSITM GSVRPSVEQF HKYLPWFLND RPNIKCPKGG LAAYSTSVNL TS DGQVLAS RFMAYHKPLK NSQDYTEALR AARELAANIT ADLRKVPGTD PAFEVFPYTI TNVFYEQYLT ILPEGLFMLS LCL VPTFAV SCLLLGLDLR SGLLNLLSIV MILVDTVGFM ALWGISYNAV SLINLVSAVG MSVEFVSHIT RSFAISTKPT WLER AKEAT ISMGSAVFAG VAMTNLPGIL VLGLAKAQLI QIFFFRLNLL ITLLGLLHGL VFLPVILSYV GPDVNPAL |
-Macromolecule #5: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 5 / Number of copies: 5 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #6: (3~{R},4~{S})-1-(4-fluorophenyl)-3-[(3~{S})-3-(4-fluorophenyl)-3-...
| Macromolecule | Name: (3~{R},4~{S})-1-(4-fluorophenyl)-3-[(3~{S})-3-(4-fluorophenyl)-3-oxidanyl-propyl]-4-(4-hydroxyphenyl)azetidin-2-one type: ligand / ID: 6 / Number of copies: 1 / Formula: H56 |
|---|---|
| Molecular weight | Theoretical: 409.425 Da |
| Chemical component information | 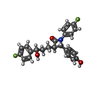 ChemComp-H56: |
-Macromolecule #7: CHOLESTEROL
| Macromolecule | Name: CHOLESTEROL / type: ligand / ID: 7 / Number of copies: 4 / Formula: CLR |
|---|---|
| Molecular weight | Theoretical: 386.654 Da |
| Chemical component information |  ChemComp-CLR: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.4 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: OTHER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.58 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 2204129 |
|---|---|
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller


















