[English] 日本語
 Yorodumi
Yorodumi- EMDB-29837: Human Oct4 bound to nucleosome with human nMatn1 sequence (focuse... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
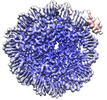
| Title | Human Oct4 bound to nucleosome with human nMatn1 sequence (focused refinement of nucleosome) | |||||||||
 Map data Map data | nMatn1 nucleosome | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Oct4 / Nucleosome / Histone / nMatn1 DNA / DNA BINDING PROTEIN-DNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationstructural constituent of chromatin / nucleosome / protein heterodimerization activity / DNA binding / nucleoplasm / nucleus Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.3 Å | |||||||||
 Authors Authors | Sinha KK / Bilokapic S / Du Y / Malik D / Halic M | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2023 Journal: Nature / Year: 2023Title: Histone modifications regulate pioneer transcription factor cooperativity. Authors: Kalyan K Sinha / Silvija Bilokapic / Yongming Du / Deepshikha Malik / Mario Halic /  Abstract: Pioneer transcription factors have the ability to access DNA in compacted chromatin. Multiple transcription factors can bind together to a regulatory element in a cooperative way, and cooperation ...Pioneer transcription factors have the ability to access DNA in compacted chromatin. Multiple transcription factors can bind together to a regulatory element in a cooperative way, and cooperation between the pioneer transcription factors OCT4 (also known as POU5F1) and SOX2 is important for pluripotency and reprogramming. However, the molecular mechanisms by which pioneer transcription factors function and cooperate on chromatin remain unclear. Here we present cryo-electron microscopy structures of human OCT4 bound to a nucleosome containing human LIN28B or nMATN1 DNA sequences, both of which bear multiple binding sites for OCT4. Our structural and biochemistry data reveal that binding of OCT4 induces changes to the nucleosome structure, repositions the nucleosomal DNA and facilitates cooperative binding of additional OCT4 and of SOX2 to their internal binding sites. The flexible activation domain of OCT4 contacts the N-terminal tail of histone H4, altering its conformation and thus promoting chromatin decompaction. Moreover, the DNA-binding domain of OCT4 engages with the N-terminal tail of histone H3, and post-translational modifications at H3K27 modulate DNA positioning and affect transcription factor cooperativity. Thus, our findings suggest that the epigenetic landscape could regulate OCT4 activity to ensure proper cell programming. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_29837.map.gz emd_29837.map.gz | 228 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-29837-v30.xml emd-29837-v30.xml emd-29837.xml emd-29837.xml | 23 KB 23 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_29837.png emd_29837.png | 241.9 KB | ||
| Filedesc metadata |  emd-29837.cif.gz emd-29837.cif.gz | 6.5 KB | ||
| Others |  emd_29837_half_map_1.map.gz emd_29837_half_map_1.map.gz emd_29837_half_map_2.map.gz emd_29837_half_map_2.map.gz | 194.1 MB 194.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-29837 http://ftp.pdbj.org/pub/emdb/structures/EMD-29837 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29837 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-29837 | HTTPS FTP |
-Validation report
| Summary document |  emd_29837_validation.pdf.gz emd_29837_validation.pdf.gz | 914.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_29837_full_validation.pdf.gz emd_29837_full_validation.pdf.gz | 914.1 KB | Display | |
| Data in XML |  emd_29837_validation.xml.gz emd_29837_validation.xml.gz | 15.5 KB | Display | |
| Data in CIF |  emd_29837_validation.cif.gz emd_29837_validation.cif.gz | 18.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29837 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29837 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29837 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-29837 | HTTPS FTP |
-Related structure data
| Related structure data |  8g86MC  8g87C  8g88C  8g8bC  8g8eC  8g8gC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_29837.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_29837.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | nMatn1 nucleosome | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.805 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #1
| File | emd_29837_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
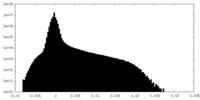
| Projections & Slices |
| ||||||||||||
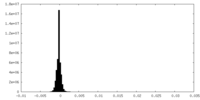
| Density Histograms |
-Half map: #2
| File | emd_29837_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
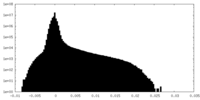
| Projections & Slices |
| ||||||||||||
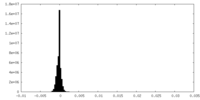
| Density Histograms |
- Sample components
Sample components
-Entire : Nucleosome with Human nMatn1 sequence
| Entire | Name: Nucleosome with Human nMatn1 sequence |
|---|---|
| Components |
|
-Supramolecule #1: Nucleosome with Human nMatn1 sequence
| Supramolecule | Name: Nucleosome with Human nMatn1 sequence / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism: |
-Macromolecule #1: Histone H3
| Macromolecule | Name: Histone H3 / type: protein_or_peptide / ID: 1 / Details: Histone H3.2|Xenopus laevis / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: |
| Molecular weight | Theoretical: 15.30393 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: ARTKQTARKS TGGKAPRKQL ATKAARKSAP ATGGVKKPHR YRPGTVALRE IRRYQKSTEL LIRKLPFQRL VREIAQDFKT DLRFQSSAV MALQEASEAY LVALFEDTNL CAIHAKRVTI MPKDIQLARR IRGERA UniProtKB: Histone H3.2 |
-Macromolecule #2: Histone H4
| Macromolecule | Name: Histone H4 / type: protein_or_peptide / ID: 2 / Details: Histone H4|Xenopus laevis / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: |
| Molecular weight | Theoretical: 11.263231 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SGRGKGGKGL GKGGAKRHRK VLRDNIQGIT KPAIRRLARR GGVKRISGLI YEETRGVLKV FLENVIRDAV TYTEHAKRKT VTAMDVVYA LKRQGRTLYG FGG UniProtKB: Histone H4 |
-Macromolecule #3: Histone H2A
| Macromolecule | Name: Histone H2A / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: |
| Molecular weight | Theoretical: 13.978241 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: SGRGKQGGKT RAKAKTRSSR AGLQFPVGRV HRLLRKGNYA ERVGAGAPVY LAAVLEYLTA EILELAGNAA RDNKKTRIIP RHLQLAVRN DEELNKLLGR VTIAQGGVLP NIQSVLLPKK TESSKSAKSK UniProtKB: Histone H2A |
-Macromolecule #4: Histone H2B
| Macromolecule | Name: Histone H2B / type: protein_or_peptide / ID: 4 / Details: Histone H2B 1.1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism: |
| Molecular weight | Theoretical: 13.524752 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: AKSAPAPKKG SKKAVTKTQK KDGKKRRKTR KESYAIYVYK VLKQVHPDTG ISSKAMSIMN SFVNDVFERI AGEASRLAHY NKRSTITSR EIQTAVRLLL PGELAKHAVS EGTKAVTKYT SAK UniProtKB: Histone H2B 1.1 |
-Macromolecule #5: nMatn1 DNA (top stand)
| Macromolecule | Name: nMatn1 DNA (top stand) / type: dna / ID: 5 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 56.93359 KDa |
| Sequence | String: (DA)(DC)(DA)(DT)(DG)(DC)(DA)(DC)(DA)(DC) (DA)(DT)(DG)(DC)(DT)(DA)(DA)(DT)(DA)(DT) (DA)(DT)(DG)(DC)(DA)(DC)(DA)(DC)(DA) (DA)(DT)(DG)(DC)(DA)(DC)(DA)(DC)(DA)(DG) (DG) (DT)(DT)(DA)(DA)(DT)(DA) ...String: (DA)(DC)(DA)(DT)(DG)(DC)(DA)(DC)(DA)(DC) (DA)(DT)(DG)(DC)(DT)(DA)(DA)(DT)(DA)(DT) (DA)(DT)(DG)(DC)(DA)(DC)(DA)(DC)(DA) (DA)(DT)(DG)(DC)(DA)(DC)(DA)(DC)(DA)(DG) (DG) (DT)(DT)(DA)(DA)(DT)(DA)(DT)(DA) (DT)(DA)(DC)(DA)(DC)(DA)(DT)(DA)(DC)(DA) (DC)(DA) (DC)(DA)(DC)(DA)(DT)(DG)(DC) (DA)(DC)(DA)(DC)(DA)(DC)(DA)(DC)(DG)(DT) (DG)(DC)(DA) (DC)(DA)(DC)(DA)(DT)(DA) (DT)(DA)(DT)(DG)(DC)(DA)(DC)(DA)(DT)(DG) (DC)(DA)(DT)(DG) (DC)(DA)(DC)(DA)(DC) (DA)(DC)(DG)(DT)(DA)(DT)(DA)(DT)(DG)(DC) (DA)(DC)(DA)(DC)(DA) (DC)(DA)(DT)(DG) (DC)(DA)(DC)(DA)(DT)(DG)(DC)(DA)(DT)(DG) (DC)(DG)(DC)(DA)(DC)(DA) (DT)(DA)(DG) (DT)(DC)(DA)(DC)(DA)(DC)(DA)(DC)(DA)(DT) (DG)(DC)(DA)(DC)(DA)(DC)(DA) (DT)(DT) (DA)(DG)(DC)(DA)(DT)(DA)(DT)(DG)(DC)(DA) (DT)(DA)(DC)(DA)(DC)(DA)(DT)(DA) (DC) (DA)(DT)(DG)(DC)(DA) |
-Macromolecule #6: nMatn1 DNA (bottom strand)
| Macromolecule | Name: nMatn1 DNA (bottom strand) / type: dna / ID: 6 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 57.889855 KDa |
| Sequence | String: (DT)(DG)(DC)(DA)(DT)(DG)(DT)(DA)(DT)(DG) (DT)(DG)(DT)(DA)(DT)(DG)(DC)(DA)(DT)(DA) (DT)(DG)(DC)(DT)(DA)(DA)(DT)(DG)(DT) (DG)(DT)(DG)(DC)(DA)(DT)(DG)(DT)(DG)(DT) (DG) (DT)(DG)(DA)(DC)(DT)(DA) ...String: (DT)(DG)(DC)(DA)(DT)(DG)(DT)(DA)(DT)(DG) (DT)(DG)(DT)(DA)(DT)(DG)(DC)(DA)(DT)(DA) (DT)(DG)(DC)(DT)(DA)(DA)(DT)(DG)(DT) (DG)(DT)(DG)(DC)(DA)(DT)(DG)(DT)(DG)(DT) (DG) (DT)(DG)(DA)(DC)(DT)(DA)(DT)(DG) (DT)(DG)(DC)(DG)(DC)(DA)(DT)(DG)(DC)(DA) (DT)(DG) (DT)(DG)(DC)(DA)(DT)(DG)(DT) (DG)(DT)(DG)(DT)(DG)(DC)(DA)(DT)(DA)(DT) (DA)(DC)(DG) (DT)(DG)(DT)(DG)(DT)(DG) (DC)(DA)(DT)(DG)(DC)(DA)(DT)(DG)(DT)(DG) (DC)(DA)(DT)(DA) (DT)(DA)(DT)(DG)(DT) (DG)(DT)(DG)(DC)(DA)(DC)(DG)(DT)(DG)(DT) (DG)(DT)(DG)(DT)(DG) (DC)(DA)(DT)(DG) (DT)(DG)(DT)(DG)(DT)(DG)(DT)(DA)(DT)(DG) (DT)(DG)(DT)(DA)(DT)(DA) (DT)(DA)(DT) (DT)(DA)(DA)(DC)(DC)(DT)(DG)(DT)(DG)(DT) (DG)(DC)(DA)(DT)(DT)(DG)(DT) (DG)(DT) (DG)(DC)(DA)(DT)(DA)(DT)(DA)(DT)(DT)(DA) (DG)(DC)(DA)(DT)(DG)(DT)(DG)(DT) (DG) (DC)(DA)(DT)(DG)(DT) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2 mg/mL | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7.5 Component:
Details: 50 mM HEPES pH 7.5, 1 mM DTT | |||||||||
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE | |||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 283 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Average electron dose: 50.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.7000000000000001 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: EMDB MAP EMDB ID: |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.3 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 450000 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller













 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)