+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | CX3CR1 nucleosome and wild type PU.1 complex | |||||||||
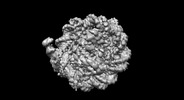
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | nucleosome / transcription factor / transcription / chromatin binding protein-DNA complex / DNA BINDING PROTEIN-DNA complex / TRANSCRIPTION-DNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of myeloid dendritic cell chemotaxis / anatomical structure regression / follicular B cell differentiation / positive regulation of antifungal innate immune response / regulation of myeloid progenitor cell differentiation / pro-T cell differentiation / negative regulation of neutrophil degranulation / germinal center B cell differentiation / immature B cell differentiation / myeloid leukocyte differentiation ...positive regulation of myeloid dendritic cell chemotaxis / anatomical structure regression / follicular B cell differentiation / positive regulation of antifungal innate immune response / regulation of myeloid progenitor cell differentiation / pro-T cell differentiation / negative regulation of neutrophil degranulation / germinal center B cell differentiation / immature B cell differentiation / myeloid leukocyte differentiation / apoptotic process involved in blood vessel morphogenesis / positive regulation of microglial cell mediated cytotoxicity / granulocyte differentiation / lymphocyte differentiation / endothelial to hematopoietic transition / negative regulation of adipose tissue development / negative regulation of MHC class II biosynthetic process / pericyte cell differentiation / lymphoid progenitor cell differentiation / myeloid dendritic cell differentiation / vasculature development / regulation of DNA-binding transcription factor activity / oncogene-induced cell senescence / negative regulation of protein localization to chromatin / positive regulation of p38MAPK cascade / positive regulation of B cell differentiation / NFAT protein binding / somatic stem cell population maintenance / macrophage differentiation / negative regulation of megakaryocyte differentiation / cis-regulatory region sequence-specific DNA binding / protein localization to CENP-A containing chromatin / Chromatin modifying enzymes / Replacement of protamines by nucleosomes in the male pronucleus / CENP-A containing nucleosome / Packaging Of Telomere Ends / lipopolysaccharide-mediated signaling pathway / Recognition and association of DNA glycosylase with site containing an affected purine / Cleavage of the damaged purine / Deposition of new CENPA-containing nucleosomes at the centromere / nucleosomal DNA binding / Recognition and association of DNA glycosylase with site containing an affected pyrimidine / Cleavage of the damaged pyrimidine / protein sequestering activity / Inhibition of DNA recombination at telomere / telomere organization / Meiotic synapsis / Interleukin-7 signaling / RNA Polymerase I Promoter Opening / epigenetic regulation of gene expression / Assembly of the ORC complex at the origin of replication / SUMOylation of chromatin organization proteins / transforming growth factor beta receptor signaling pathway / Regulation of endogenous retroelements by the Human Silencing Hub (HUSH) complex / transcription initiation-coupled chromatin remodeling / DNA methylation / Condensation of Prophase Chromosomes / erythrocyte differentiation / SIRT1 negatively regulates rRNA expression / Chromatin modifications during the maternal to zygotic transition (MZT) / ERCC6 (CSB) and EHMT2 (G9a) positively regulate rRNA expression / HCMV Late Events / innate immune response in mucosa / PRC2 methylates histones and DNA / Regulation of endogenous retroelements by KRAB-ZFP proteins / Defective pyroptosis / Regulation of endogenous retroelements by Piwi-interacting RNAs (piRNAs) / HDACs deacetylate histones / Nonhomologous End-Joining (NHEJ) / RNA Polymerase I Promoter Escape / Transcriptional regulation by small RNAs / Formation of the beta-catenin:TCF transactivating complex / RUNX1 regulates genes involved in megakaryocyte differentiation and platelet function / NoRC negatively regulates rRNA expression / Activated PKN1 stimulates transcription of AR (androgen receptor) regulated genes KLK2 and KLK3 / G2/M DNA damage checkpoint / HDMs demethylate histones / B-WICH complex positively regulates rRNA expression / DNA Damage/Telomere Stress Induced Senescence / heterochromatin formation / PKMTs methylate histone lysines / Metalloprotease DUBs / Meiotic recombination / DNA-binding transcription repressor activity, RNA polymerase II-specific / Pre-NOTCH Transcription and Translation / positive regulation of miRNA transcription / histone deacetylase binding / RMTs methylate histone arginines / Activation of anterior HOX genes in hindbrain development during early embryogenesis / HCMV Early Events / Transcriptional regulation of granulopoiesis / structural constituent of chromatin / UCH proteinases / antimicrobial humoral immune response mediated by antimicrobial peptide / nucleosome / nucleosome assembly / antibacterial humoral response / Recruitment and ATM-mediated phosphorylation of repair and signaling proteins at DNA double strand breaks / chromatin organization / RUNX1 regulates transcription of genes involved in differentiation of HSCs Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /   | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.85 Å | |||||||||
 Authors Authors | Lian T / Guan R / Bai Y | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2024 Journal: Nat Struct Mol Biol / Year: 2024Title: Structural mechanism of synergistic targeting of the CX3CR1 nucleosome by PU.1 and C/EBPα. Authors: Tengfei Lian / Ruifang Guan / Bing-Rui Zhou / Yawen Bai /  Abstract: Pioneer transcription factors are vital for cell fate changes. PU.1 and C/EBPα work together to regulate hematopoietic stem cell differentiation. However, how they recognize in vivo nucleosomal DNA ...Pioneer transcription factors are vital for cell fate changes. PU.1 and C/EBPα work together to regulate hematopoietic stem cell differentiation. However, how they recognize in vivo nucleosomal DNA targets remains elusive. Here we report the structures of the nucleosome containing the mouse genomic CX3CR1 enhancer DNA and its complexes with PU.1 alone and with both PU.1 and the C/EBPα DNA binding domain. Our structures reveal that PU.1 binds the DNA motif at the exit linker, shifting 17 bp of DNA into the core region through interactions with H2A, unwrapping ~20 bp of nucleosomal DNA. C/EBPα binding, aided by PU.1's repositioning, unwraps ~25 bp of entry DNA. The PU.1 Q218H mutation, linked to acute myeloid leukemia, disrupts PU.1-H2A interactions. PU.1 and C/EBPα jointly displace linker histone H1 and open the H1-condensed nucleosome array. Our study unveils how two pioneer factors can work cooperatively to open closed chromatin by altering DNA positioning in the nucleosome. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_28629.map.gz emd_28629.map.gz | 43.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-28629-v30.xml emd-28629-v30.xml emd-28629.xml emd-28629.xml | 21.5 KB 21.5 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_28629.png emd_28629.png | 40.8 KB | ||
| Filedesc metadata |  emd-28629.cif.gz emd-28629.cif.gz | 6.7 KB | ||
| Others |  emd_28629_half_map_1.map.gz emd_28629_half_map_1.map.gz emd_28629_half_map_2.map.gz emd_28629_half_map_2.map.gz | 41.1 MB 41.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-28629 http://ftp.pdbj.org/pub/emdb/structures/EMD-28629 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28629 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-28629 | HTTPS FTP |
-Validation report
| Summary document |  emd_28629_validation.pdf.gz emd_28629_validation.pdf.gz | 899.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_28629_full_validation.pdf.gz emd_28629_full_validation.pdf.gz | 898.8 KB | Display | |
| Data in XML |  emd_28629_validation.xml.gz emd_28629_validation.xml.gz | 11.9 KB | Display | |
| Data in CIF |  emd_28629_validation.cif.gz emd_28629_validation.cif.gz | 13.9 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28629 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28629 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28629 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-28629 | HTTPS FTP |
-Related structure data
| Related structure data |  8evhMC  8eviC  8evjC  8sypC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_28629.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_28629.map.gz / Format: CCP4 / Size: 52.7 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.056 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_28629_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_28629_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : nucleosome PU.1 complex
| Entire | Name: nucleosome PU.1 complex |
|---|---|
| Components |
|
-Supramolecule #1: nucleosome PU.1 complex
| Supramolecule | Name: nucleosome PU.1 complex / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#8 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Histone H3.1
| Macromolecule | Name: Histone H3.1 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 15.437167 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MARTKQTARK STGGKAPRKQ LATKAARKSA PATGGVKKPH RYRPGTVALR EIRRYQKSTE LLIRKLPFQR LVREIAQDFK TDLRFQSSA VMALQEACEA YLVGLFEDTN LCAIHAKRVT IMPKDIQLAR RIRGERA UniProtKB: Histone H3.1 |
-Macromolecule #2: Histone H4
| Macromolecule | Name: Histone H4 / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 11.394426 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSGRGKGGKG LGKGGAKRHR KVLRDNIQGI TKPAIRRLAR RGGVKRISGL IYEETRGVLK VFLENVIRDA VTYTEHAKRK TVTAMDVVY ALKRQGRTLY GFGG UniProtKB: Histone H4 |
-Macromolecule #3: Histone H2A type 2-C
| Macromolecule | Name: Histone H2A type 2-C / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 14.017428 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSGRGKQGGK ARAKAKSRSS RAGLQFPVGR VHRLLRKGNY AERVGAGAPV YMAAVLEYLT AEILELAGNA ARDNKKTRII PRHLQLAIR NDEELNKLLG KVTIAQGGVL PNIQAVLLPK KTESHKAKSK UniProtKB: Histone H2A type 2-C |
-Macromolecule #4: Histone H2B type 2-E
| Macromolecule | Name: Histone H2B type 2-E / type: protein_or_peptide / ID: 4 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 13.951239 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MPEPAKSAPA PKKGSKKAVT KAQKKDGKKR KRSRKESYSI YVYKVLKQVH PDTGISSKAM GIMNSFVNDI FERIAGEASR LAHYNKRST ITSREIQTAV RLLLPGELAK HAVSEGTKAV TKYTSSK UniProtKB: Histone H2B type 2-E |
-Macromolecule #7: Single-chain variable fragment
| Macromolecule | Name: Single-chain variable fragment / type: protein_or_peptide / ID: 7 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 29.030146 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKSSHHHHHH ENLYFQSNAM EVQLQQSGPE LVEPGTSVKM PCKASGYTFT SYTIQWVKQT PRQGLEWIGY IYPYNAGTKY NEKFKGKAT LTSDKSSSTV YMELSSLTSE DSAVYYCARK SSRLRSTLDY WGQGTSVTVS SGGGGSGGGG SGGGGSMDIK M TQSPSSMH ...String: MKSSHHHHHH ENLYFQSNAM EVQLQQSGPE LVEPGTSVKM PCKASGYTFT SYTIQWVKQT PRQGLEWIGY IYPYNAGTKY NEKFKGKAT LTSDKSSSTV YMELSSLTSE DSAVYYCARK SSRLRSTLDY WGQGTSVTVS SGGGGSGGGG SGGGGSMDIK M TQSPSSMH ASLGERVTIT CKASQDIRSY LSWYQQKPWK SPKTLIYYAT SLADGVPSRF SGSGSGQDFS LTINNLESDD TA TYYCLQH GESPYTFGSG TKLEIKRA |
-Macromolecule #8: Transcription factor PU.1
| Macromolecule | Name: Transcription factor PU.1 / type: protein_or_peptide / ID: 8 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 32.81975 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MGSSHHHHHH SSGMLQACKM EGFSLTAPPS DDLVTYDSEL YQRPMHDYYS FVGSDGESHS DHYWDFSAHH VHNNEFENFP ENHFTELQS VQPPQLQQLY RHMELEQMHV LDTPMVPPHT GLSHQVSYMP RMCFPYQTLS PAHQQSSDEE EGERQSPPLE V SDGEADGL ...String: MGSSHHHHHH SSGMLQACKM EGFSLTAPPS DDLVTYDSEL YQRPMHDYYS FVGSDGESHS DHYWDFSAHH VHNNEFENFP ENHFTELQS VQPPQLQQLY RHMELEQMHV LDTPMVPPHT GLSHQVSYMP RMCFPYQTLS PAHQQSSDEE EGERQSPPLE V SDGEADGL EPGPGLLHGE TGSKKKIRLY QFLLDLLRSG DMKDSIWWVD KDKGTFQFSS KHKEALAHRW GIQKGNRKKM TY QKMARAL RNYGKTGEVK KVKKKLTYQF SGEVLGRGGL AERRLPPH UniProtKB: Transcription factor PU.1 |
-Macromolecule #5: DNA (162-MER)
| Macromolecule | Name: DNA (162-MER) / type: dna / ID: 5 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 50.043855 KDa |
| Sequence | String: (DT)(DA)(DG)(DG)(DT)(DG)(DC)(DA)(DG)(DG) (DG)(DC)(DC)(DT)(DC)(DT)(DC)(DG)(DG)(DC) (DT)(DG)(DC)(DT)(DG)(DA)(DT)(DC)(DT) (DT)(DC)(DA)(DG)(DC)(DT)(DG)(DG)(DT)(DT) (DG) (DC)(DT)(DG)(DA)(DG)(DA) ...String: (DT)(DA)(DG)(DG)(DT)(DG)(DC)(DA)(DG)(DG) (DG)(DC)(DC)(DT)(DC)(DT)(DC)(DG)(DG)(DC) (DT)(DG)(DC)(DT)(DG)(DA)(DT)(DC)(DT) (DT)(DC)(DA)(DG)(DC)(DT)(DG)(DG)(DT)(DT) (DG) (DC)(DT)(DG)(DA)(DG)(DA)(DG)(DT) (DT)(DG)(DC)(DA)(DG)(DC)(DA)(DT)(DT)(DG) (DC)(DT) (DG)(DA)(DG)(DT)(DC)(DT)(DT) (DA)(DG)(DC)(DA)(DA)(DT)(DG)(DG)(DA)(DT) (DA)(DC)(DT) (DT)(DC)(DC)(DC)(DG)(DA) (DT)(DT)(DC)(DC)(DC)(DC)(DT)(DC)(DA)(DC) (DA)(DA)(DA)(DA) (DA)(DT)(DA)(DG)(DG) (DT)(DC)(DA)(DG)(DT)(DC)(DT)(DG)(DT)(DC) (DT)(DG)(DG)(DC)(DT) (DA)(DG)(DT)(DT) (DC)(DT)(DG)(DT)(DA)(DC)(DT)(DT)(DG)(DC) (DA)(DG)(DA)(DC)(DA)(DC) (DA)(DG)(DG) (DG)(DC)(DA)(DT)(DG)(DT)(DG)(DG)(DG)(DG) (DT)(DT)(DC)(DC)(DT)(DA)(DT) (DT)(DT) |
-Macromolecule #6: DNA (162-MER)
| Macromolecule | Name: DNA (162-MER) / type: dna / ID: 6 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 49.965957 KDa |
| Sequence | String: (DA)(DA)(DA)(DT)(DA)(DG)(DG)(DA)(DA)(DC) (DC)(DC)(DC)(DA)(DC)(DA)(DT)(DG)(DC)(DC) (DC)(DT)(DG)(DT)(DG)(DT)(DC)(DT)(DG) (DC)(DA)(DA)(DG)(DT)(DA)(DC)(DA)(DG)(DA) (DA) (DC)(DT)(DA)(DG)(DC)(DC) ...String: (DA)(DA)(DA)(DT)(DA)(DG)(DG)(DA)(DA)(DC) (DC)(DC)(DC)(DA)(DC)(DA)(DT)(DG)(DC)(DC) (DC)(DT)(DG)(DT)(DG)(DT)(DC)(DT)(DG) (DC)(DA)(DA)(DG)(DT)(DA)(DC)(DA)(DG)(DA) (DA) (DC)(DT)(DA)(DG)(DC)(DC)(DA)(DG) (DA)(DC)(DA)(DG)(DA)(DC)(DT)(DG)(DA)(DC) (DC)(DT) (DA)(DT)(DT)(DT)(DT)(DT)(DG) (DT)(DG)(DA)(DG)(DG)(DG)(DG)(DA)(DA)(DT) (DC)(DG)(DG) (DG)(DA)(DA)(DG)(DT)(DA) (DT)(DC)(DC)(DA)(DT)(DT)(DG)(DC)(DT)(DA) (DA)(DG)(DA)(DC) (DT)(DC)(DA)(DG)(DC) (DA)(DA)(DT)(DG)(DC)(DT)(DG)(DC)(DA)(DA) (DC)(DT)(DC)(DT)(DC) (DA)(DG)(DC)(DA) (DA)(DC)(DC)(DA)(DG)(DC)(DT)(DG)(DA)(DA) (DG)(DA)(DT)(DC)(DA)(DG) (DC)(DA)(DG) (DC)(DC)(DG)(DA)(DG)(DA)(DG)(DG)(DC)(DC) (DC)(DT)(DG)(DC)(DA)(DC)(DC) (DT)(DA) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.3 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 53.8 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: NONE |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.85 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 89583 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)