+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Glycine and glutamate bound Human GluN1a-GluN2D NMDA receptor | |||||||||
 Map data Map data | Composite map | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationexcitatory chemical synaptic transmission / regulation of sensory perception of pain / cellular response to L-glutamate / Synaptic adhesion-like molecules / propylene metabolic process / response to glycine / voltage-gated monoatomic cation channel activity / regulation of monoatomic cation transmembrane transport / NMDA glutamate receptor activity / Assembly and cell surface presentation of NMDA receptors ...excitatory chemical synaptic transmission / regulation of sensory perception of pain / cellular response to L-glutamate / Synaptic adhesion-like molecules / propylene metabolic process / response to glycine / voltage-gated monoatomic cation channel activity / regulation of monoatomic cation transmembrane transport / NMDA glutamate receptor activity / Assembly and cell surface presentation of NMDA receptors / Neurexins and neuroligins / NMDA selective glutamate receptor complex / glutamate-gated calcium ion channel activity / calcium ion transmembrane import into cytosol / protein heterotetramerization / glutamate binding / positive regulation of reactive oxygen species biosynthetic process / glycine binding / positive regulation of calcium ion transport into cytosol / Negative regulation of NMDA receptor-mediated neuronal transmission / startle response / Unblocking of NMDA receptors, glutamate binding and activation / monoatomic cation transmembrane transport / monoatomic cation transport / regulation of neuronal synaptic plasticity / positive regulation of excitatory postsynaptic potential / ligand-gated monoatomic ion channel activity / Long-term potentiation / excitatory synapse / calcium ion homeostasis / synaptic cleft / glutamate-gated receptor activity / presynaptic active zone membrane / ligand-gated monoatomic ion channel activity involved in regulation of presynaptic membrane potential / EPHB-mediated forward signaling / ionotropic glutamate receptor signaling pathway / Ras activation upon Ca2+ influx through NMDA receptor / excitatory postsynaptic potential / hippocampal mossy fiber to CA3 synapse / positive regulation of synaptic transmission, glutamatergic / adult locomotory behavior / synaptic membrane / synaptic transmission, glutamatergic / transmitter-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / regulation of membrane potential / long-term synaptic potentiation / postsynaptic density membrane / visual learning / regulation of synaptic plasticity / brain development / terminal bouton / synaptic vesicle / signaling receptor activity / amyloid-beta binding / RAF/MAP kinase cascade / chemical synaptic transmission / postsynaptic membrane / response to ethanol / dendritic spine / postsynaptic density / calmodulin binding / neuron projection / glutamatergic synapse / synapse / calcium ion binding / dendrite / protein-containing complex binding / endoplasmic reticulum membrane / cell surface / positive regulation of transcription by RNA polymerase II / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.38 Å | |||||||||
 Authors Authors | Kang H / Furukawa H | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2022 Journal: Mol Cell / Year: 2022Title: Structural insights into assembly and function of GluN1-2C, GluN1-2A-2C, and GluN1-2D NMDARs. Authors: Tsung-Han Chou / Hyunook Kang / Noriko Simorowski / Stephen F Traynelis / Hiro Furukawa /  Abstract: Neurotransmission mediated by diverse subtypes of N-methyl-D-aspartate receptors (NMDARs) is fundamental for basic brain functions and development as well as neuropsychiatric diseases and disorders. ...Neurotransmission mediated by diverse subtypes of N-methyl-D-aspartate receptors (NMDARs) is fundamental for basic brain functions and development as well as neuropsychiatric diseases and disorders. NMDARs are glycine- and glutamate-gated ion channels that exist as heterotetramers composed of obligatory GluN1 and GluN2(A-D) and/or GluN3(A-B). The GluN2C and GluN2D subunits form ion channels with distinct properties and spatio-temporal expression patterns. Here, we provide the structures of the agonist-bound human GluN1-2C NMDAR in the presence and absence of the GluN2C-selective positive allosteric potentiator (PAM), PYD-106, the agonist-bound GluN1-2A-2C tri-heteromeric NMDAR, and agonist-bound GluN1-2D NMDARs by single-particle electron cryomicroscopy. Our analysis shows unique inter-subunit and domain arrangements of the GluN2C NMDARs, which contribute to functional regulation and formation of the PAM binding pocket and is distinct from GluN2D NMDARs. Our findings here provide the fundamental blueprint to study GluN2C- and GluN2D-containing NMDARs, which are uniquely involved in neuropsychiatric disorders. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27957.map.gz emd_27957.map.gz | 227.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27957-v30.xml emd-27957-v30.xml emd-27957.xml emd-27957.xml | 23.9 KB 23.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_27957.png emd_27957.png | 75 KB | ||
| Others |  emd_27957_additional_1.map.gz emd_27957_additional_1.map.gz emd_27957_additional_2.map.gz emd_27957_additional_2.map.gz emd_27957_additional_3.map.gz emd_27957_additional_3.map.gz | 229.9 MB 230.2 MB 229.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27957 http://ftp.pdbj.org/pub/emdb/structures/EMD-27957 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27957 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27957 | HTTPS FTP |
-Validation report
| Summary document |  emd_27957_validation.pdf.gz emd_27957_validation.pdf.gz | 422.2 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_27957_full_validation.pdf.gz emd_27957_full_validation.pdf.gz | 421.8 KB | Display | |
| Data in XML |  emd_27957_validation.xml.gz emd_27957_validation.xml.gz | 7.1 KB | Display | |
| Data in CIF |  emd_27957_validation.cif.gz emd_27957_validation.cif.gz | 8.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27957 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27957 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27957 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27957 | HTTPS FTP |
-Related structure data
| Related structure data |  8e96MC  8e92C  8e93C  8e94C  8e97C  8e98C  8e99C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_27957.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27957.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Composite map | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.856 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: ECD Local
| File | emd_27957_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | ECD_Local | ||||||||||||
| Projections & Slices |
| ||||||||||||
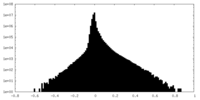
| Density Histograms |
-Additional map: LBD Local
| File | emd_27957_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | LBD_Local | ||||||||||||
| Projections & Slices |
| ||||||||||||
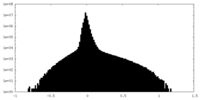
| Density Histograms |
-Additional map: TMD Local
| File | emd_27957_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | TMD_Local | ||||||||||||
| Projections & Slices |
| ||||||||||||
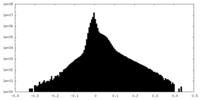
| Density Histograms |
- Sample components
Sample components
-Entire : Hetero-tetrameric GluN1a-GluN2D NMDA receptors
| Entire | Name: Hetero-tetrameric GluN1a-GluN2D NMDA receptors |
|---|---|
| Components |
|
-Supramolecule #1: Hetero-tetrameric GluN1a-GluN2D NMDA receptors
| Supramolecule | Name: Hetero-tetrameric GluN1a-GluN2D NMDA receptors / type: complex / Chimera: Yes / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Glutamate receptor ionotropic, NMDA 1
| Macromolecule | Name: Glutamate receptor ionotropic, NMDA 1 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 93.07825 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: RAASDPKIVN IGAVLSTRKH EQMFREAVNQ ANKRHGSWKI QLNATSVTHK PNAIQMALSV CEDLISSQVY AILVSHPPTP NDHFTPTPV SYTAGFYRIP VLGLTTRMSI YSDKSIHLSF LRTVPPYSHQ SSVWFEMMRV YSWNHIILLV SDDHEGRAAQ K RLETLLEE ...String: RAASDPKIVN IGAVLSTRKH EQMFREAVNQ ANKRHGSWKI QLNATSVTHK PNAIQMALSV CEDLISSQVY AILVSHPPTP NDHFTPTPV SYTAGFYRIP VLGLTTRMSI YSDKSIHLSF LRTVPPYSHQ SSVWFEMMRV YSWNHIILLV SDDHEGRAAQ K RLETLLEE RESKAEKVLQ FDPGTKNVTA LLMEAKELEA RVIILSASED DAATVYRAAA MLNMTGSGYV WLVGEREISG NA LRYAPDG ILGLQLINGK NESAHISDAV GVVAQAVHEL LEKENITDPP RGCVGNTNIW KTGPLFKRVL MSSKYADGVT GRV EFNEDG DRKFANYSIM NLQNRKLVQV GIYNGTHVIP NDRKIIWPGG ETEKPRGYQM STRLKIVTIH QEPFVYVKPT LSDG TCKEE FTVNGDPVKK VICTGPNDTS PGSPRHTVPQ CCYGFCIDLL IKLARTMNFT YEVHLVADGK FGTQERVNNS NKKEW NGMM GELLSGQADM IVAPLTINNE RAQYIEFSKP FKYQGLTILV KKEIPRSTLD SFMQPFQSTL WLLVGLSVHV VAVMLY LLD RFSPFGRFKV NSEEEEEDAL TLSSAMWFSW GVLLNSGIGE GAPRSFSARI LGMVWAGFAM IIVASYTANL AAFLVLD RP EERITGINDP RLRNPSDKFI YATVKQSSVD IYFRRQVELS TMYRHMEKHN YESAAEAIQA VRDNKLHAFI WDSAVLEF E ASQKCDLVTT GELFFRSGFG IGMRKDSPWK QNVSLSILKS HENGFMEDLD KTWVRYQECD SRSNAPATLT FENMAGVFM LVAGGIVAGI FLIFIEIAYK RHKDANGAQ |
-Macromolecule #2: Glutamate receptor ionotropic, NMDA 2D
| Macromolecule | Name: Glutamate receptor ionotropic, NMDA 2D / type: protein_or_peptide / ID: 2 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 96.760492 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: WSHPQFEKGG GSGGGSGGSA WSHPQFEKGA LVPRGFPEEA PGPGGAGGPG GGLGGARPLN VALVFSGPAY AAEAARLGPA VAAAVRSPG LDVRPVALVL NGSDPRSLVL QLCDLLSGLR VHGVVFEDDS RAPAVAPILD FLSAQTSLPI VAVHGGAALV L TPKEKGST ...String: WSHPQFEKGG GSGGGSGGSA WSHPQFEKGA LVPRGFPEEA PGPGGAGGPG GGLGGARPLN VALVFSGPAY AAEAARLGPA VAAAVRSPG LDVRPVALVL NGSDPRSLVL QLCDLLSGLR VHGVVFEDDS RAPAVAPILD FLSAQTSLPI VAVHGGAALV L TPKEKGST FLQLGSSTEQ QLQVIFEVLE EYDWTSFVAV TTRAPGHRAF LSYIEVLTDG SLVGWEHRGA LTLDPGAGEA VL SAQLRSV SAQIRLLFCA REEAEPVFRA AEEAGLTGSG YVWFMVGPQL AGGGGSGAPG EPPLLPGGAP LPAGLFAVRS AGW RDDLAR RVAAGVAVVA RGAQALLRDY GFLPELGHDC RAQNRTHRGE SLHRYFMNIT WDNRDYSFNE DGFLVNPSLV VISL TRDRT WEVVGSWEQQ TLRLKYPLWS RYGRFLQPVD DTQHLTVATL EERPFVIVEP ADPISGTCIR DSVPCRSQLN RTHSP PPDA PRPEKRCCKG FCIDILKRLA HTIGFSYDLY LVTNGKHGKK IDGVWNGMIG EVFYQRADMA IGSLTINEER SEIVDF SVP FVETGISVMV ARSNGTVSPS AFLEPYSPAV WVMMFVMCLT VVAVTVFIFE YLSPVGYNRS LATGKRPGGS TFTIGKS IW LLWALVFNNS VPVENPRGTT SKIMVLVWAF FAVIFLASYT ANLAAFMIQE EYVDTVSGLS DRKFQRPQEQ YPPLKFGT V PNGSTEKNIR SNYPDMHSYM VRYNQPRVEE ALTQLKAGKL DAFIYDAAVL NYMARKDEGC KLVTIGSGKV FATTGYGIA LHKGSRWKRP IDLALLQFLG DDEIEMLERL WLSGICHNDK IEVMSSKLDI DNMAGVFYML LVAMGLSLLV FAWEHLVYWR LRHCLGP |
-Macromolecule #3: GLYCINE
| Macromolecule | Name: GLYCINE / type: ligand / ID: 3 / Number of copies: 2 / Formula: GLY |
|---|---|
| Molecular weight | Theoretical: 75.067 Da |
| Chemical component information |  ChemComp-GLY: |
-Macromolecule #4: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 4 / Number of copies: 6 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #5: GLUTAMIC ACID
| Macromolecule | Name: GLUTAMIC ACID / type: ligand / ID: 5 / Number of copies: 2 / Formula: GLU |
|---|---|
| Molecular weight | Theoretical: 147.129 Da |
| Chemical component information |  ChemComp-GLU: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 4 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 85 % / Chamber temperature: 285 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 74.7 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.4 µm / Nominal defocus min: 0.6 µm |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller
























 Z
Z Y
Y X
X