[English] 日本語
 Yorodumi
Yorodumi- EMDB-27458: Cryo-EM structure of mouse PrP23-144 amyloid fibrils (polymorph 1) -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of mouse PrP23-144 amyloid fibrils (polymorph 1) | |||||||||
 Map data Map data | Cryo-EM structure of mouse PrP23-144 amyloid fibrils (polymorph 1) | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | moPrP23-144 / amyloid / prion diseases / PROTEIN FIBRIL | |||||||||
| Function / homology |  Function and homology information Function and homology informationInsertion of tail-anchored proteins into the endoplasmic reticulum membrane / negative regulation of amyloid precursor protein catabolic process / lamin binding / regulation of glutamate receptor signaling pathway / regulation of calcium ion import across plasma membrane / aspartic-type endopeptidase inhibitor activity / glycosaminoglycan binding / ATP-dependent protein binding / negative regulation of interleukin-17 production / type 5 metabotropic glutamate receptor binding ...Insertion of tail-anchored proteins into the endoplasmic reticulum membrane / negative regulation of amyloid precursor protein catabolic process / lamin binding / regulation of glutamate receptor signaling pathway / regulation of calcium ion import across plasma membrane / aspartic-type endopeptidase inhibitor activity / glycosaminoglycan binding / ATP-dependent protein binding / negative regulation of interleukin-17 production / type 5 metabotropic glutamate receptor binding / negative regulation of dendritic spine maintenance / cupric ion binding / regulation of potassium ion transmembrane transport / response to copper ion / nucleobase-containing compound metabolic process / negative regulation of calcineurin-NFAT signaling cascade / negative regulation of T cell receptor signaling pathway / negative regulation of interleukin-2 production / activation of protein kinase activity / negative regulation of amyloid-beta formation / negative regulation of activated T cell proliferation / cuprous ion binding / response to amyloid-beta / negative regulation of type II interferon production / negative regulation of long-term synaptic potentiation / intracellular copper ion homeostasis / positive regulation of protein targeting to membrane / response to cadmium ion / regulation of peptidyl-tyrosine phosphorylation / side of membrane / inclusion body / cellular response to copper ion / neuron projection maintenance / positive regulation of calcium-mediated signaling / negative regulation of protein phosphorylation / molecular function activator activity / positive regulation of protein localization to plasma membrane / molecular condensate scaffold activity / protein destabilization / negative regulation of DNA-binding transcription factor activity / protein homooligomerization / terminal bouton / cellular response to amyloid-beta / positive regulation of peptidyl-tyrosine phosphorylation / positive regulation of neuron apoptotic process / cellular response to xenobiotic stimulus / regulation of protein localization / signaling receptor activity / amyloid-beta binding / protein-folding chaperone binding / microtubule binding / protease binding / nuclear membrane / response to oxidative stress / transmembrane transporter binding / molecular adaptor activity / postsynaptic density / learning or memory / membrane raft / copper ion binding / dendrite / protein-containing complex binding / negative regulation of apoptotic process / Golgi apparatus / cell surface / endoplasmic reticulum / identical protein binding / membrane / metal ion binding / plasma membrane / cytosol Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | helical reconstruction / cryo EM / Resolution: 3.92 Å | |||||||||
 Authors Authors | Li Q / Surewicz WK | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2022 Journal: Nat Struct Mol Biol / Year: 2022Title: Cryo-EM structure of disease-related prion fibrils provides insights into seeding barriers. Authors: Qiuye Li / Christopher P Jaroniec / Witold K Surewicz /  Abstract: One of the least understood aspects of prion diseases is the structure of infectious prion protein aggregates. Here we report a high-resolution cryo-EM structure of amyloid fibrils formed by human ...One of the least understood aspects of prion diseases is the structure of infectious prion protein aggregates. Here we report a high-resolution cryo-EM structure of amyloid fibrils formed by human prion protein with the Y145Stop mutation that is associated with a familial prion disease. This structural insight allows us not only to explain previous biochemical findings, but also provides direct support for the conformational adaptability model of prion transmissibility barriers. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27458.map.gz emd_27458.map.gz | 2.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27458-v30.xml emd-27458-v30.xml emd-27458.xml emd-27458.xml | 13.6 KB 13.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_27458.png emd_27458.png | 174.1 KB | ||
| Filedesc metadata |  emd-27458.cif.gz emd-27458.cif.gz | 5.1 KB | ||
| Others |  emd_27458_half_map_1.map.gz emd_27458_half_map_1.map.gz emd_27458_half_map_2.map.gz emd_27458_half_map_2.map.gz | 987.7 KB 984.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27458 http://ftp.pdbj.org/pub/emdb/structures/EMD-27458 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27458 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27458 | HTTPS FTP |
-Validation report
| Summary document |  emd_27458_validation.pdf.gz emd_27458_validation.pdf.gz | 562 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_27458_full_validation.pdf.gz emd_27458_full_validation.pdf.gz | 561.6 KB | Display | |
| Data in XML |  emd_27458_validation.xml.gz emd_27458_validation.xml.gz | 7.3 KB | Display | |
| Data in CIF |  emd_27458_validation.cif.gz emd_27458_validation.cif.gz | 8.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27458 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27458 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27458 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27458 | HTTPS FTP |
-Related structure data
| Related structure data |  8djaMC  7rl4C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_27458.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27458.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of mouse PrP23-144 amyloid fibrils (polymorph 1) | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.828 Å | ||||||||||||||||||||||||||||||||||||
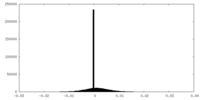
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Cryo-EM structure of mouse PrP23-144 amyloid fibrils (polymorph 1)
| File | emd_27458_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of mouse PrP23-144 amyloid fibrils (polymorph 1) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Cryo-EM structure of mouse PrP23-144 amyloid fibrils (polymorph 1)
| File | emd_27458_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of mouse PrP23-144 amyloid fibrils (polymorph 1) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Amyloid fibrils formed by moPrP23-144
| Entire | Name: Amyloid fibrils formed by moPrP23-144 |
|---|---|
| Components |
|
-Supramolecule #1: Amyloid fibrils formed by moPrP23-144
| Supramolecule | Name: Amyloid fibrils formed by moPrP23-144 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Major prion protein
| Macromolecule | Name: Major prion protein / type: protein_or_peptide / ID: 1 / Number of copies: 20 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 12.398579 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: GSKKRPKPGG WNTGGSRYPG QGSPGGNRYP PQGGTWGQPH GGGWGQPHGG SWGQPHGGSW GQPHGGGWGQ GGGTHNQWNK PSKPKTNLK HVAGAAAAGA VVGGLGGYML GSAMSRPMIH FGND UniProtKB: Major prion protein |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Concentration | 0.5 mg/mL |
|---|---|
| Buffer | pH: 6.5 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 52.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 4.74 Å Applied symmetry - Helical parameters - Δ&Phi: -2.36 ° Applied symmetry - Helical parameters - Axial symmetry: C2 (2 fold cyclic) Resolution.type: BY AUTHOR / Resolution: 3.92 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 10628 |
|---|---|
| Startup model | Type of model: NONE |
| Final angle assignment | Type: NOT APPLICABLE |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)