[English] 日本語
 Yorodumi
Yorodumi- EMDB-27399: Cryo-electron microscopy structure of Neisseria gonorrhoeae multi... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-electron microscopy structure of Neisseria gonorrhoeae multidrug efflux pump MtrD with CASP peptide complex | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationefflux transmembrane transporter activity / xenobiotic transmembrane transporter activity / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Neisseria gonorrhoeae (bacteria) Neisseria gonorrhoeae (bacteria) | |||||||||
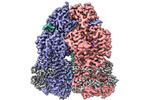
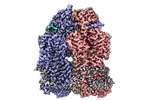
| Method | single particle reconstruction / cryo EM / Resolution: 2.95 Å | |||||||||
 Authors Authors | Lyu M / Yu EW | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Microbiol Spectr / Year: 2022 Journal: Microbiol Spectr / Year: 2022Title: Structural Basis of Peptide-Based Antimicrobial Inhibition of a Resistance-Nodulation-Cell Division Multidrug Efflux Pump. Authors: Meinan Lyu / Julio C Ayala / Isabella Chirakos / Chih-Chia Su / William M Shafer / Edward W Yu /  Abstract: Bacterial efflux pumps in the resistance-nodulation-cell division (RND) family of Gram-negative bacteria contribute significantly to the development of antimicrobial resistance by many pathogens. In ...Bacterial efflux pumps in the resistance-nodulation-cell division (RND) family of Gram-negative bacteria contribute significantly to the development of antimicrobial resistance by many pathogens. In this study, we selected the MtrD transporter protein of Neisseria gonorrhoeae as it is the sole RND pump possessed by this strictly human pathogen and can export multiple antimicrobials, including antibiotics, bile salts, detergents, dyes, and antimicrobial peptides. Using knowledge from our previously published structures of MtrD in the presence or absence of bound antibiotics as a model and the known ability of MtrCDE to export cationic antimicrobial peptides, we hypothesized that cationic peptides could be accommodated within MtrD binding sites. Furthermore, we thought that MtrD-bound peptides lacking antibacterial action could sensitize bacteria to an antibiotic normally exported by the MtrCDE efflux pump or other similar RND-type pumps possessed by different Gram-negative bacteria. We now report the identification of a novel nonantimicrobial cyclic cationic antimicrobial peptide, which we termed CASP (ationic ntibiotic-ensitizing eptide). By single-particle cryo-electron microscopy, we found that CASP binds within the periplasmic cleft region of MtrD using overlapping and distinct amino acid contact sites that interact with another cyclic peptide (colistin) or a linear human cationic antimicrobial peptide derived from human LL-37. While CASP could not sensitize Neisseria gonorrhoeae to an antibiotic (novobiocin) that is a substrate for RND pumps, it could do so against multiple Gram-negative, rod-shaped bacteria. We propose that CASP (or future derivatives) could serve as an adjuvant for the antibiotic treatment of certain Gram-negative infections previously thwarted by RND transporters. RND efflux pumps can export numerous antimicrobials that enter Gram-negative bacteria, and their action can reduce the efficacy of antibiotics and provide decreased susceptibility to various host antimicrobials. Here, we identified a ationic ntibiotic-ensitizing eptide (CASP) that binds within the periplasmic cleft of an RND transporter protein (MtrD) produced by Neisseria gonorrhoeae. Surprisingly, CASP was able to render rod-shaped Gram-negative bacteria, but not gonococci, susceptible to an antibiotic that is a substrate for the gonococcal MtrCDE efflux pump. CASP (or its future derivatives) could be used as an adjuvant to treat infections for which RND efflux contributes to multidrug resistance. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_27399.map.gz emd_27399.map.gz | 97.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-27399-v30.xml emd-27399-v30.xml emd-27399.xml emd-27399.xml | 15.9 KB 15.9 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_27399_fsc.xml emd_27399_fsc.xml | 13.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_27399.png emd_27399.png | 123.1 KB | ||
| Others |  emd_27399_half_map_1.map.gz emd_27399_half_map_1.map.gz emd_27399_half_map_2.map.gz emd_27399_half_map_2.map.gz | 95.7 MB 95.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-27399 http://ftp.pdbj.org/pub/emdb/structures/EMD-27399 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27399 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-27399 | HTTPS FTP |
-Validation report
| Summary document |  emd_27399_validation.pdf.gz emd_27399_validation.pdf.gz | 974.6 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_27399_full_validation.pdf.gz emd_27399_full_validation.pdf.gz | 974.2 KB | Display | |
| Data in XML |  emd_27399_validation.xml.gz emd_27399_validation.xml.gz | 17.9 KB | Display | |
| Data in CIF |  emd_27399_validation.cif.gz emd_27399_validation.cif.gz | 23.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27399 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27399 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27399 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-27399 | HTTPS FTP |
-Related structure data
| Related structure data |  8deuMC  8devC  8dewC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_27399.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_27399.map.gz / Format: CCP4 / Size: 103 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_27399_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
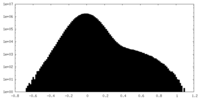
| Projections & Slices |
| ||||||||||||
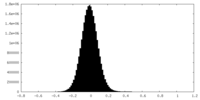
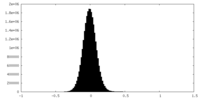
| Density Histograms |
-Half map: #1
| File | emd_27399_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
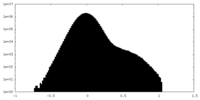
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Multidrug efflux pump MtrD with CASP peptide complex
| Entire | Name: Multidrug efflux pump MtrD with CASP peptide complex |
|---|---|
| Components |
|
-Supramolecule #1: Multidrug efflux pump MtrD with CASP peptide complex
| Supramolecule | Name: Multidrug efflux pump MtrD with CASP peptide complex / type: complex / Chimera: Yes / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Neisseria gonorrhoeae (bacteria) Neisseria gonorrhoeae (bacteria) |
| Recombinant expression | Organism:  |
-Macromolecule #1: Efflux pump membrane transporter
| Macromolecule | Name: Efflux pump membrane transporter / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Neisseria gonorrhoeae (bacteria) Neisseria gonorrhoeae (bacteria) |
| Molecular weight | Theoretical: 113.932469 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MAKFFIDRPI FAWVISIFII AAGIFGIKSL PVSQYPSVAA PTITLHAIYP GASAQVMEGS VLSVIERNMN GVEGLDYMST SADSSGSGS VSLTFTPDTD ENLAQVEVQN KLSEVLSTLP ATVQQYGVTV SKARSNFLMI VMLSSDVQST EEMNDYAQRN V VPELQRIE ...String: MAKFFIDRPI FAWVISIFII AAGIFGIKSL PVSQYPSVAA PTITLHAIYP GASAQVMEGS VLSVIERNMN GVEGLDYMST SADSSGSGS VSLTFTPDTD ENLAQVEVQN KLSEVLSTLP ATVQQYGVTV SKARSNFLMI VMLSSDVQST EEMNDYAQRN V VPELQRIE GVGQVRLFGA QRAMRIWVDP KKLQNYNLSF ADVGSALSAQ NIQISAGSIG SLPAVRGQTV TATVTAQGQL GT AEEFGNV ILRANTDGSN IYLKDVAKVG LGMEDYSSST RLNGVNTTGM AVMLSNSGNA MATAKAVKER LAVLEKYFPQ GMS WKTPYD TSKFVEISIE KVIHTLIEAM VLVFVVMYLF LQNIRYTLIP TIVVPISLLG GFAFISYMGM SINVLTMFAM ILVI GIVVD DAIVVVENVE RIMAGEGLPP KEATKKAMGQ ISGAVIGITA VLISVFVPLA MFSGAAGNIY KQFALTMASS IAFSA FLAL TLTPALCATM LKTIPKGHHE EKKGFFGWFN KKFDSWTHGY EGRVAKVLRK TFRMMVVYIG LAVVGVFLFM RLPTSF LPT EDQGFVMVSV QLPAGATKER TDATLAQVTQ LAKSIPEIEN IITVSGFSFS GSGQNMAMGF AILKDWNERT ASGSDAV AV AGKLTGMMMG TLKDGFGIAV VPPPILELGN GSGLSINLQD RNNTGHTALL AKRNELIQKM RASGLFDPST VRAGGLED S PQLKIDINRA AAAAQGVSFA DIRTALASAL SSSYVSDFPN QGRLQRVMVQ ADGDARMQPA DILNLTVPNS SGIAVPLSS IATVSWQMGT EQSVRFNGYP AMELSGSPAT GVSTGQAMEA VQKMVDELGS GYSLEWGGQS REEAKGGSQT IALYALAAVA VFLVLAALY ESWSIPLAVL LVMPLGLAGA AAGVTGRNLF EGLLGSVPSF ANDIYFQVGF VTVMGLSAKN AILIIEFAKD L QAQGKSAV EAALEAARLR FRPIIMTSFA FILGVVPLYI AGGASSASQR AIGTTVFWGM LIGTLLSVFL VPLFYVVVRK FF KETAHEH EMAVRHASKA GITGSDDKQY |
-Macromolecule #2: CASP peptide
| Macromolecule | Name: CASP peptide / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Neisseria gonorrhoeae (bacteria) Neisseria gonorrhoeae (bacteria) |
| Molecular weight | Theoretical: 967.055 Da |
| Recombinant expression | Organism:  |
| Sequence | String: CTNEDGKPC |
-Macromolecule #3: PHOSPHATIDYLETHANOLAMINE
| Macromolecule | Name: PHOSPHATIDYLETHANOLAMINE / type: ligand / ID: 3 / Number of copies: 27 / Formula: PTY |
|---|---|
| Molecular weight | Theoretical: 734.039 Da |
| Chemical component information |  ChemComp-PTY: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.7 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 1 / Number real images: 5188 / Average exposure time: 2.6 sec. / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.75 µm / Nominal defocus min: 0.75 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






 Z
Z Y
Y X
X