+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
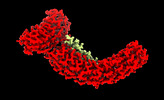
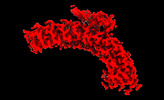
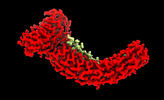
| Title | Class 2 of erythrocyte ankyrin-1 complex (Composite map) | |||||||||
 Map data Map data | Main map used for model fitting; composite map of local refinements (EMDs: 26978, 26979, 26975, 26973, 26972, 26974) aligned to the class 2 consensus refinement. | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationnitric oxide transmembrane transporter activity / metanephric descending thin limb development / metanephric proximal straight tubule development / metanephric proximal convoluted tubule segment 2 development / metanephric glomerulus vasculature development / cerebrospinal fluid secretion / methylammonium transmembrane transport / Defective RHAG causes regulator type Rh-null hemolytic anemia (RHN) / Rhesus blood group biosynthesis / methylammonium transmembrane transporter activity ...nitric oxide transmembrane transporter activity / metanephric descending thin limb development / metanephric proximal straight tubule development / metanephric proximal convoluted tubule segment 2 development / metanephric glomerulus vasculature development / cerebrospinal fluid secretion / methylammonium transmembrane transport / Defective RHAG causes regulator type Rh-null hemolytic anemia (RHN) / Rhesus blood group biosynthesis / methylammonium transmembrane transporter activity / lipid digestion / Rhesus glycoproteins mediate ammonium transport. / cellular response to salt stress / renal water transport / glycerol transmembrane transporter activity / corticotropin secretion / secretory granule organization / carbon dioxide transmembrane transport / carbon dioxide transmembrane transporter activity / renal water absorption / positive regulation of saliva secretion / Passive transport by Aquaporins / glycerol transmembrane transport / water transmembrane transporter activity / ammonium homeostasis / establishment or maintenance of actin cytoskeleton polarity / pancreatic juice secretion / cellular response to mercury ion / lateral ventricle development / spectrin-associated cytoskeleton / hemoglobin metabolic process / positive regulation of organelle organization / potassium ion transmembrane transporter activity / : / intracellular water homeostasis / protein-glutamine gamma-glutamyltransferase activity / maintenance of epithelial cell apical/basal polarity / ammonium transmembrane transport / leak channel activity / water transport / water channel activity / pH elevation / Defective SLC4A1 causes hereditary spherocytosis type 4 (HSP4), distal renal tubular acidosis (dRTA) and dRTA with hemolytic anemia (dRTA-HA) / negative regulation of urine volume / transepithelial water transport / glomerular filtration / NrCAM interactions / Bicarbonate transporters / Neurofascin interactions / intracellular monoatomic ion homeostasis / ankyrin-1 complex / intracellularly cGMP-activated cation channel activity / plasma membrane phospholipid scrambling / CHL1 interactions / monoatomic anion transmembrane transporter activity / ammonium channel activity / chloride:bicarbonate antiporter activity / camera-type eye morphogenesis / cytoskeletal anchor activity / fibroblast migration / multicellular organismal-level water homeostasis / solute:inorganic anion antiporter activity / cellular homeostasis / cellular hyperosmotic response / hyperosmotic response / renal water homeostasis / bicarbonate transport / cell volume homeostasis / bicarbonate transmembrane transporter activity / M band / positive regulation of fibroblast migration / monoatomic anion transport / Interaction between L1 and Ankyrins / inorganic cation transmembrane transport / chloride transport / odontogenesis / chloride transmembrane transporter activity / nitric oxide transport / ankyrin binding / negative regulation of glycolytic process through fructose-6-phosphate / hemoglobin binding / spectrin binding / cortical cytoskeleton / cGMP-mediated signaling / exocytosis / erythrocyte maturation / potassium channel activity / brush border / transmembrane transporter activity / axolemma / cellular response to nitric oxide / erythrocyte development / endoplasmic reticulum to Golgi vesicle-mediated transport / COPI-mediated anterograde transport / cellular response to retinoic acid / protein-membrane adaptor activity / cellular response to cAMP / spleen development / sensory perception of pain / chloride transmembrane transport Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /  human (human) human (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.9 Å | |||||||||
 Authors Authors | Vallese F / Kim K / Yen LY / Johnston JD / Noble AJ / Cali T / Clarke OB | |||||||||
| Funding support | 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2022 Journal: Nat Struct Mol Biol / Year: 2022Title: Architecture of the human erythrocyte ankyrin-1 complex. Authors: Francesca Vallese / Kookjoo Kim / Laura Y Yen / Jake D Johnston / Alex J Noble / Tito Calì / Oliver Biggs Clarke /   Abstract: The stability and shape of the erythrocyte membrane is provided by the ankyrin-1 complex, but how it tethers the spectrin-actin cytoskeleton to the lipid bilayer and the nature of its association ...The stability and shape of the erythrocyte membrane is provided by the ankyrin-1 complex, but how it tethers the spectrin-actin cytoskeleton to the lipid bilayer and the nature of its association with the band 3 anion exchanger and the Rhesus glycoproteins remains unknown. Here we present structures of ankyrin-1 complexes purified from human erythrocytes. We reveal the architecture of a core complex of ankyrin-1, the Rhesus proteins RhAG and RhCE, the band 3 anion exchanger, protein 4.2, glycophorin A and glycophorin B. The distinct T-shaped conformation of membrane-bound ankyrin-1 facilitates recognition of RhCE and, unexpectedly, the water channel aquaporin-1. Together, our results uncover the molecular details of ankyrin-1 association with the erythrocyte membrane, and illustrate the mechanism of ankyrin-mediated membrane protein clustering. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_26988.map.gz emd_26988.map.gz | 28.1 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-26988-v30.xml emd-26988-v30.xml emd-26988.xml emd-26988.xml | 45 KB 45 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_26988_fsc.xml emd_26988_fsc.xml | 15.9 KB | Display |  FSC data file FSC data file |
| Images |  emd_26988.png emd_26988.png | 89.8 KB | ||
| Others |  emd_26988_additional_1.map.gz emd_26988_additional_1.map.gz emd_26988_additional_2.map.gz emd_26988_additional_2.map.gz emd_26988_additional_3.map.gz emd_26988_additional_3.map.gz emd_26988_additional_4.map.gz emd_26988_additional_4.map.gz emd_26988_additional_5.map.gz emd_26988_additional_5.map.gz emd_26988_additional_6.map.gz emd_26988_additional_6.map.gz emd_26988_half_map_1.map.gz emd_26988_half_map_1.map.gz emd_26988_half_map_2.map.gz emd_26988_half_map_2.map.gz | 39.1 MB 325.5 MB 326.3 MB 328 MB 172.5 MB 2.9 MB 322.9 MB 322.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-26988 http://ftp.pdbj.org/pub/emdb/structures/EMD-26988 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26988 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26988 | HTTPS FTP |
-Validation report
| Summary document |  emd_26988_validation.pdf.gz emd_26988_validation.pdf.gz | 731.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_26988_full_validation.pdf.gz emd_26988_full_validation.pdf.gz | 731.1 KB | Display | |
| Data in XML |  emd_26988_validation.xml.gz emd_26988_validation.xml.gz | 23.6 KB | Display | |
| Data in CIF |  emd_26988_validation.cif.gz emd_26988_validation.cif.gz | 31.1 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26988 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26988 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26988 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26988 | HTTPS FTP |
-Related structure data
| Related structure data |  8cteMC  7uz3C  7uzeC  7uzqC  7uzsC  7uzuC  7uzvC  7v07C  7v0kC  7v0mC  7v0qC  7v0sC  7v0tC  7v0uC  7v0xC  7v0yC  7v19C  8crqC  8crrC  8crtC  8cs9C  8cslC  8csvC  8cswC  8csxC  8csyC  8ct2C  8ct3C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_26988.map.gz / Format: CCP4 / Size: 347.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_26988.map.gz / Format: CCP4 / Size: 347.6 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Main map used for model fitting; composite map of local refinements (EMDs: 26978, 26979, 26975, 26973, 26972, 26974) aligned to the class 2 consensus refinement. | ||||||||||||||||||||
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: Composite map of local refinements (unsharpened, unfiltered), aligned...
| File | emd_26988_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Composite map of local refinements (unsharpened, unfiltered), aligned to the global consensus refinement provided in additional maps. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Composite map of local refinements (unsharpened, unfiltered), aligned...
| File | emd_26988_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Composite map of local refinements (unsharpened, unfiltered), aligned to the global consensus refinement provided in additional maps. Lowpass filtered at 4A. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Composite map of local refinements, aligned to the...
| File | emd_26988_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Composite map of local refinements, aligned to the global consensus refinement provided in additional maps. Density modified using phenix.resolve_cryo_em. Lowpass filtered at 4A. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Class 2 consensus refinement. B-factor sharpened.
| File | emd_26988_additional_4.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Class 2 consensus refinement. B-factor sharpened. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Class 2 consensus refinement, unsharpened.
| File | emd_26988_additional_5.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Class 2 consensus refinement, unsharpened. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: Mask used for FSC calculation.
| File | emd_26988_additional_6.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Mask used for FSC calculation. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 1 (of global consensus refinement; main...
| File | emd_26988_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 (of global consensus refinement; main map is a composite of local refinements) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 2 (of global consensus refinement; main...
| File | emd_26988_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 (of global consensus refinement; main map is a composite of local refinements) | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Class 1 of erythrocyte ankyrin complex (composite map)
+Supramolecule #1: Class 1 of erythrocyte ankyrin complex (composite map)
+Macromolecule #1: Ankyrin-1
+Macromolecule #2: Band 3 anion transport protein
+Macromolecule #3: Protein 4.2
+Macromolecule #4: Blood group Rh(CE) polypeptide
+Macromolecule #5: Ammonium transporter Rh type A
+Macromolecule #6: Glycophorin-A
+Macromolecule #7: Aquaporin-1
+Macromolecule #8: CHOLESTEROL
+Macromolecule #9: Digitonin
+Macromolecule #10: 2-acetamido-2-deoxy-beta-D-glucopyranose
+Macromolecule #11: [(2R)-2-octanoyloxy-3-[oxidanyl-[(1R,2R,3S,4R,5R,6S)-2,3,6-tris(o...
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 8 mg/mL |
|---|---|
| Buffer | pH: 7.4 Details: Final gel filtration buffer contained 0.05% w/v digitonin, 130 mM KCl, 20 mM HEPES, pH 7.4, 1 mM ATP, 1 mM MgCl2, 1 mM PMSF. Peak fractions were concentrated to 8 mg/mL, and 0.01% w/v ...Details: Final gel filtration buffer contained 0.05% w/v digitonin, 130 mM KCl, 20 mM HEPES, pH 7.4, 1 mM ATP, 1 mM MgCl2, 1 mM PMSF. Peak fractions were concentrated to 8 mg/mL, and 0.01% w/v glycyrrhizic acid was added immediately prior to vitrification. |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV / Details: 4-6 seconds, wait time 30 seconds. |
| Details | Ankyrin complex mixture purified from digitonin-solubilized erythrocyte ghost membranes |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Number grids imaged: 2 / Number real images: 14464 / Average exposure time: 2.5 sec. / Average electron dose: 58.0 e/Å2 / Details: Two grids were imaged in a single session. |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.5 µm |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller












































 Z
Z Y
Y X
X