[English] 日本語
 Yorodumi
Yorodumi- EMDB-26581: Human Kv4.2-KChIP2-DPP6 channel complex in an open state, intrace... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human Kv4.2-KChIP2-DPP6 channel complex in an open state, intracellular region | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | potassium channel complex / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationER retention sequence binding / positive regulation of voltage-gated potassium channel activity / Kv4.2-KChIP2 channel complex / clustering of voltage-gated potassium channels / A-type (transient outward) potassium channel activity / Phase 1 - inactivation of fast Na+ channels / positive regulation of potassium ion export across plasma membrane / voltage-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / potassium channel complex / membrane repolarization during cardiac muscle cell action potential ...ER retention sequence binding / positive regulation of voltage-gated potassium channel activity / Kv4.2-KChIP2 channel complex / clustering of voltage-gated potassium channels / A-type (transient outward) potassium channel activity / Phase 1 - inactivation of fast Na+ channels / positive regulation of potassium ion export across plasma membrane / voltage-gated monoatomic ion channel activity involved in regulation of postsynaptic membrane potential / potassium channel complex / membrane repolarization during cardiac muscle cell action potential / potassium ion export across plasma membrane / membrane repolarization / Voltage gated Potassium channels / regulation of potassium ion transmembrane transport / postsynaptic specialization membrane / anchoring junction / action potential / regulation of heart contraction / neuronal cell body membrane / voltage-gated potassium channel activity / potassium channel activity / plasma membrane raft / locomotor rhythm / detection of calcium ion / GABA-ergic synapse / potassium channel regulator activity / neuronal action potential / potassium ion transmembrane transport / voltage-gated potassium channel complex / sensory perception of pain / muscle contraction / potassium ion transport / protein homooligomerization / cellular response to hypoxia / chemical synaptic transmission / postsynaptic membrane / perikaryon / transmembrane transporter binding / dendritic spine / neuronal cell body / glutamatergic synapse / synapse / calcium ion binding / dendrite / protein-containing complex binding / signal transduction / identical protein binding / metal ion binding / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.33 Å | |||||||||
 Authors Authors | Zhao H / Dai Y / Lee CH | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2022 Journal: Mol Cell / Year: 2022Title: Activation and closed-state inactivation mechanisms of the human voltage-gated K4 channel complexes. Authors: Wenlei Ye / Hongtu Zhao / Yaxin Dai / Yingdi Wang / Yu-Hua Lo / Lily Yeh Jan / Chia-Hsueh Lee /  Abstract: The voltage-gated ion channel activity depends on both activation (transition from the resting state to the open state) and inactivation. Inactivation is a self-restraint mechanism to limit ion ...The voltage-gated ion channel activity depends on both activation (transition from the resting state to the open state) and inactivation. Inactivation is a self-restraint mechanism to limit ion conduction and is as crucial to membrane excitability as activation. Inactivation can occur when the channel is open or closed. Although open-state inactivation is well understood, the molecular basis of closed-state inactivation has remained elusive. We report cryo-EM structures of human K4.2 channel complexes in inactivated, open, and closed states. Closed-state inactivation of K4 involves an unprecedented symmetry breakdown for pore closure by only two of the four S4-S5 linkers, distinct from known mechanisms of open-state inactivation. We further capture K4 in a putative resting state, revealing how voltage sensor movements control the pore. Moreover, our structures provide insights regarding channel modulation by KChIP2 and DPP6 auxiliary subunits. Our findings elucidate mechanisms of closed-state inactivation and voltage-dependent activation of the K4 channel. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_26581.map.gz emd_26581.map.gz | 398.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-26581-v30.xml emd-26581-v30.xml emd-26581.xml emd-26581.xml | 15.1 KB 15.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_26581.png emd_26581.png | 107.1 KB | ||
| Filedesc metadata |  emd-26581.cif.gz emd-26581.cif.gz | 5.6 KB | ||
| Others |  emd_26581_half_map_1.map.gz emd_26581_half_map_1.map.gz emd_26581_half_map_2.map.gz emd_26581_half_map_2.map.gz | 390.9 MB 390.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-26581 http://ftp.pdbj.org/pub/emdb/structures/EMD-26581 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26581 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26581 | HTTPS FTP |
-Validation report
| Summary document |  emd_26581_validation.pdf.gz emd_26581_validation.pdf.gz | 896.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_26581_full_validation.pdf.gz emd_26581_full_validation.pdf.gz | 896.4 KB | Display | |
| Data in XML |  emd_26581_validation.xml.gz emd_26581_validation.xml.gz | 18.1 KB | Display | |
| Data in CIF |  emd_26581_validation.cif.gz emd_26581_validation.cif.gz | 21.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26581 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26581 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26581 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26581 | HTTPS FTP |
-Related structure data
| Related structure data |  7ukhMC  7uk5C  7ukcC  7ukdC  7ukeC  7ukfC  7ukgC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_26581.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_26581.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.826 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
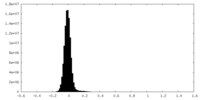
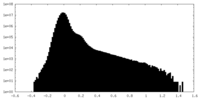
-Half map: #1
| File | emd_26581_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
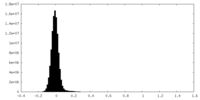
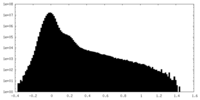
-Half map: #2
| File | emd_26581_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Human Kv4.2-KChIP2-DPP6 channel complex in an open state, intrace...
| Entire | Name: Human Kv4.2-KChIP2-DPP6 channel complex in an open state, intracellular region |
|---|---|
| Components |
|
-Supramolecule #1: Human Kv4.2-KChIP2-DPP6 channel complex in an open state, intrace...
| Supramolecule | Name: Human Kv4.2-KChIP2-DPP6 channel complex in an open state, intracellular region type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Potassium voltage-gated channel subfamily D member 2
| Macromolecule | Name: Potassium voltage-gated channel subfamily D member 2 / type: protein_or_peptide / ID: 1 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 59.049332 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MAAGVAAWLP FARAAAIGWM PVASGPMPAP PRQERKRTQD ALIVLNVSGT RFQTWQDTLE RYPDTLLGSS ERDFFYHPET QQYFFDRDP DIFRHILNFY RTGKLHYPRH ECISAYDEEL AFFGLIPEII GDCCYEEYKD RRRENAERLQ DDADTDTAGE S ALPTMTAR ...String: MAAGVAAWLP FARAAAIGWM PVASGPMPAP PRQERKRTQD ALIVLNVSGT RFQTWQDTLE RYPDTLLGSS ERDFFYHPET QQYFFDRDP DIFRHILNFY RTGKLHYPRH ECISAYDEEL AFFGLIPEII GDCCYEEYKD RRRENAERLQ DDADTDTAGE S ALPTMTAR QRVWRAFENP HTSTMALVFY YVTGFFIAVS VIANVVETVP CGSSPGHIKE LPCGERYAVA FFCLDTACVM IF TVEYLLR LAAAPSRYRF VRSVMSIIDV VAILPYYIGL VMTDNEDVSG AFVTLRVFRV FRIFKFSRHS QGLRILGYTL KSC ASELGF LLFSLTMAII IFATVMFYAE KGSSASKFTS IPAAFWYTIV TMTTLGYGDM VPKTIAGKIF GSICSLSGVL VIAL PVPVI VSNFSRIYHQ NQRADKRRAQ KKARLARIRA AKSGSANAYM QSKRNGLLSN QLQSSEDEQA FVSKSGSSFE TQHHH LLHC LEKTTNHEFV DEQVFEESCM EVATVNRPSS HSPSLSSQQG UniProtKB: Potassium voltage-gated channel subfamily D member 2 |
-Macromolecule #2: Isoform 2 of Kv channel-interacting protein 2
| Macromolecule | Name: Isoform 2 of Kv channel-interacting protein 2 / type: protein_or_peptide / ID: 2 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 28.975486 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MRGQGRKESL SDSRDLDGSY DQLTGHPPGP TKKALKQRFL KLLPCCGPQA LPSVSENSVD DEFELSTVCH RPEGLEQLQE QTKFTRKEL QVLYRGFKNE CPSGIVNEEN FKQIYSQFFP QGDSSTYATF LFNAFDTNHD GSVSFEDFVA GLSVILRGTV D DRLNWAFN ...String: MRGQGRKESL SDSRDLDGSY DQLTGHPPGP TKKALKQRFL KLLPCCGPQA LPSVSENSVD DEFELSTVCH RPEGLEQLQE QTKFTRKEL QVLYRGFKNE CPSGIVNEEN FKQIYSQFFP QGDSSTYATF LFNAFDTNHD GSVSFEDFVA GLSVILRGTV D DRLNWAFN LYDLNKDGCI TKEEMLDIMK SIYDMMGKYT YPALREEAPR EHVESFFQKM DRNKDGVVTI EEFIESCQKD EN IMRSMQL FDNVI UniProtKB: Kv channel-interacting protein 2 |
-Macromolecule #3: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 3 / Number of copies: 4 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Macromolecule #4: CALCIUM ION
| Macromolecule | Name: CALCIUM ION / type: ligand / ID: 4 / Number of copies: 12 / Formula: CA |
|---|---|
| Molecular weight | Theoretical: 40.078 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 85.9 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.6 µm / Nominal defocus min: 0.6 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: OTHER |
|---|---|
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 2.33 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 278343 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller














 Z
Z Y
Y X
X