[English] 日本語
 Yorodumi
Yorodumi- EMDB-26231: Yeast TRAPPII-Rab11/Ypt32 complex in the closed/closed state (foc... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Yeast TRAPPII-Rab11/Ypt32 complex in the closed/closed state (focused refinement of Trs120 middle region) | |||||||||
 Map data Map data | Map | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | complex / GTPase / Guanosine Exchange Factor / GEF / PROTEIN TRANSPORT | |||||||||
| Biological species |  | |||||||||
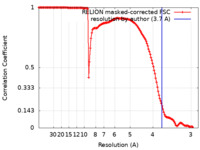
| Method | single particle reconstruction / cryo EM / Resolution: 3.7 Å | |||||||||
 Authors Authors | Bagde SR / Fromme JC | |||||||||
| Funding support |  United States, 2 items United States, 2 items
| |||||||||
 Citation Citation |  Journal: Sci Adv / Year: 2022 Journal: Sci Adv / Year: 2022Title: Structure of a TRAPPII-Rab11 activation intermediate reveals GTPase substrate selection mechanisms. Authors: Saket R Bagde / J Christopher Fromme /  Abstract: Rab1 and Rab11 are essential regulators of the eukaryotic secretory and endocytic recycling pathways. The transport protein particle (TRAPP) complexes activate these guanosine triphosphatases via ...Rab1 and Rab11 are essential regulators of the eukaryotic secretory and endocytic recycling pathways. The transport protein particle (TRAPP) complexes activate these guanosine triphosphatases via nucleotide exchange using a shared set of core subunits. The basal specificity of the TRAPP core is toward Rab1, yet the TRAPPII complex is specific for Rab11. A steric gating mechanism has been proposed to explain TRAPPII counterselection against Rab1. Here, we present cryo-electron microscopy structures of the 22-subunit TRAPPII complex from budding yeast, including a TRAPPII-Rab11 nucleotide exchange intermediate. The Trs130 subunit provides a "leg" that positions the active site distal to the membrane surface, and this leg is required for steric gating. The related TRAPPIII complex is unable to activate Rab11 because of a repulsive interaction, which TRAPPII surmounts using the Trs120 subunit as a "lid" to enclose the active site. TRAPPII also adopts an open conformation enabling Rab11 to access and exit from the active site chamber. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_26231.map.gz emd_26231.map.gz | 192.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-26231-v30.xml emd-26231-v30.xml emd-26231.xml emd-26231.xml | 22.6 KB 22.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_26231_fsc.xml emd_26231_fsc.xml | 14.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_26231.png emd_26231.png | 102.1 KB | ||
| Masks |  emd_26231_msk_1.map emd_26231_msk_1.map | 244.1 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-26231.cif.gz emd-26231.cif.gz | 5 KB | ||
| Others |  emd_26231_additional_1.map.gz emd_26231_additional_1.map.gz emd_26231_additional_2.map.gz emd_26231_additional_2.map.gz emd_26231_half_map_1.map.gz emd_26231_half_map_1.map.gz emd_26231_half_map_2.map.gz emd_26231_half_map_2.map.gz | 1.4 MB 10.4 MB 193.2 MB 194 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-26231 http://ftp.pdbj.org/pub/emdb/structures/EMD-26231 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26231 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-26231 | HTTPS FTP |
-Validation report
| Summary document |  emd_26231_validation.pdf.gz emd_26231_validation.pdf.gz | 718.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_26231_full_validation.pdf.gz emd_26231_full_validation.pdf.gz | 718 KB | Display | |
| Data in XML |  emd_26231_validation.xml.gz emd_26231_validation.xml.gz | 21.5 KB | Display | |
| Data in CIF |  emd_26231_validation.cif.gz emd_26231_validation.cif.gz | 28.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26231 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26231 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26231 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-26231 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_26231.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_26231.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Map | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.432 Å | ||||||||||||||||||||||||||||||||||||
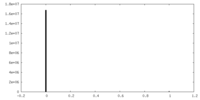
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_26231_msk_1.map emd_26231_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
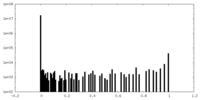
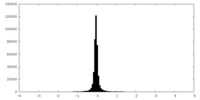
| Density Histograms |
-Additional map: RESOLVE Density Modified Map
| File | emd_26231_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | RESOLVE Density Modified Map | ||||||||||||
| Projections & Slices |
| ||||||||||||
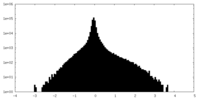
| Density Histograms |
-Additional map: PostProcessed (sharpened) masked map
| File | emd_26231_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | PostProcessed (sharpened) masked map | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Half map 1
| File | emd_26231_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 1 | ||||||||||||
| Projections & Slices |
| ||||||||||||
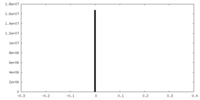
| Density Histograms |
-Half map: Half map 2
| File | emd_26231_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Half map 2 | ||||||||||||
| Projections & Slices |
| ||||||||||||
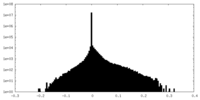
| Density Histograms |
- Sample components
Sample components
-Entire : TRAPPII complex bound to Rab11/Ypt32
| Entire | Name: TRAPPII complex bound to Rab11/Ypt32 |
|---|---|
| Components |
|
-Supramolecule #1: TRAPPII complex bound to Rab11/Ypt32
| Supramolecule | Name: TRAPPII complex bound to Rab11/Ypt32 / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#13 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 1.1 MDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 5.6 mg/mL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
| ||||||||||||
| Grid | Model: UltrAuFoil R1.2/1.3 / Support film - Material: GOLD / Support film - topology: HOLEY / Support film - Film thickness: 50 / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. | ||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV Details: The sample was incubated on the grid for 10 seconds followed by blotting for 5 seconds before plunging in liquid ethane.. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Digitization - Dimensions - Width: 5760 pixel / Digitization - Dimensions - Height: 4092 pixel / Number grids imaged: 3 / Number real images: 4998 / Average exposure time: 3.5 sec. / Average electron dose: 53.0 e/Å2 / Details: Images were collected as 50 frame movies. |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 63000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller









































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)