[English] 日本語
 Yorodumi
Yorodumi- EMDB-25193: Full-length insulin receptor bound with both site 1 binding defic... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Full-length insulin receptor bound with both site 1 binding deficient mutant insulin (A-V3E) and site 2 binding deficient mutant insulin (A-L13R) | |||||||||
 Map data Map data | Cryo-EM structure of full-length insulin receptor bound with both site 1 binding deficient mutant insulin (A-V3E) and site 2 binding deficient mutant insulin (A-L13R) | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | insulin receptor / site 1 binding deficient mutant insulin / SIGNALING PROTEIN / SIGNALING PROTEIN-HORMONE complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationSignaling by Insulin receptor / yolk / IRS activation / Insulin receptor signalling cascade / Signal attenuation / Insulin receptor recycling / 3-phosphoinositide-dependent protein kinase binding / positive regulation of glycoprotein biosynthetic process / lipoic acid binding / regulation of female gonad development ...Signaling by Insulin receptor / yolk / IRS activation / Insulin receptor signalling cascade / Signal attenuation / Insulin receptor recycling / 3-phosphoinositide-dependent protein kinase binding / positive regulation of glycoprotein biosynthetic process / lipoic acid binding / regulation of female gonad development / regulation of hydrogen peroxide metabolic process / positive regulation of meiotic cell cycle / PI5P, PP2A and IER3 Regulate PI3K/AKT Signaling / nuclear lumen / insulin-like growth factor II binding / positive regulation of developmental growth / male sex determination / exocrine pancreas development / insulin receptor complex / insulin-like growth factor I binding / insulin receptor activity / positive regulation of protein-containing complex disassembly / cargo receptor activity / dendritic spine maintenance / insulin binding / negative regulation of NAD(P)H oxidase activity / negative regulation of glycogen catabolic process / PTB domain binding / adrenal gland development / positive regulation of nitric oxide mediated signal transduction / negative regulation of fatty acid metabolic process / negative regulation of feeding behavior / Signaling by Insulin receptor / IRS activation / Insulin processing / neuronal cell body membrane / regulation of protein secretion / positive regulation of peptide hormone secretion / positive regulation of respiratory burst / positive regulation of receptor internalization / negative regulation of acute inflammatory response / Regulation of gene expression in beta cells / alpha-beta T cell activation / amyloid-beta clearance / regulation of amino acid metabolic process / regulation of embryonic development / negative regulation of respiratory burst involved in inflammatory response / insulin receptor substrate binding / negative regulation of protein secretion / positive regulation of dendritic spine maintenance / positive regulation of glycogen biosynthetic process / Synthesis, secretion, and deacylation of Ghrelin / epidermis development / regulation of protein localization to plasma membrane / fatty acid homeostasis / negative regulation of gluconeogenesis / negative regulation of lipid catabolic process / response to tumor necrosis factor / Signal attenuation / FOXO-mediated transcription of oxidative stress, metabolic and neuronal genes / COPI-mediated anterograde transport / phosphatidylinositol 3-kinase binding / heart morphogenesis / positive regulation of lipid biosynthetic process / negative regulation of oxidative stress-induced intrinsic apoptotic signaling pathway / positive regulation of insulin receptor signaling pathway / positive regulation of phosphorylation / nitric oxide-cGMP-mediated signaling / negative regulation of reactive oxygen species biosynthetic process / positive regulation of protein autophosphorylation / Insulin receptor recycling / transport vesicle / insulin-like growth factor receptor binding / dendrite membrane / neuron projection maintenance / endoplasmic reticulum-Golgi intermediate compartment membrane / positive regulation of brown fat cell differentiation / positive regulation of protein metabolic process / NPAS4 regulates expression of target genes / activation of protein kinase B activity / positive regulation of glycolytic process / Insulin receptor signalling cascade / positive regulation of mitotic nuclear division / receptor-mediated endocytosis / negative regulation of protein phosphorylation / response to nutrient levels / positive regulation of nitric-oxide synthase activity / positive regulation of cytokine production / positive regulation of long-term synaptic potentiation / acute-phase response / endosome lumen / Regulation of insulin secretion / positive regulation of D-glucose import / positive regulation of protein secretion / negative regulation of proteolysis / animal organ morphogenesis / positive regulation of cell differentiation / regulation of transmembrane transporter activity / insulin receptor binding / wound healing Similarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.1 Å | |||||||||
 Authors Authors | Bai XC / Choi E | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2022 Journal: Nat Struct Mol Biol / Year: 2022Title: Synergistic activation of the insulin receptor via two distinct sites. Authors: Jie Li / Junhee Park / John P Mayer / Kristofor J Webb / Emiko Uchikawa / Jiayi Wu / Shun Liu / Xuewu Zhang / Michael H B Stowell / Eunhee Choi / Xiao-Chen Bai /  Abstract: Insulin receptor (IR) signaling controls multiple facets of animal physiology. Maximally four insulins bind to IR at two distinct sites, termed site-1 and site-2. However, the precise functional ...Insulin receptor (IR) signaling controls multiple facets of animal physiology. Maximally four insulins bind to IR at two distinct sites, termed site-1 and site-2. However, the precise functional roles of each binding event during IR activation remain unresolved. Here, we showed that IR incompletely saturated with insulin predominantly forms an asymmetric conformation and exhibits partial activation. IR with one insulin bound adopts a Γ-shaped conformation. IR with two insulins bound assumes a Ƭ-shaped conformation. One insulin binds at site-1 and another simultaneously contacts both site-1 and site-2 in the Ƭ-shaped IR dimer. We further show that concurrent binding of four insulins to sites-1 and -2 prevents the formation of asymmetric IR and promotes the T-shaped symmetric, fully active state. Collectively, our results demonstrate how the synergistic binding of multiple insulins promotes optimal IR activation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_25193.map.gz emd_25193.map.gz | 166.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-25193-v30.xml emd-25193-v30.xml emd-25193.xml emd-25193.xml | 16.3 KB 16.3 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_25193.png emd_25193.png | 26.9 KB | ||
| Filedesc metadata |  emd-25193.cif.gz emd-25193.cif.gz | 6.2 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-25193 http://ftp.pdbj.org/pub/emdb/structures/EMD-25193 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25193 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-25193 | HTTPS FTP |
-Validation report
| Summary document |  emd_25193_validation.pdf.gz emd_25193_validation.pdf.gz | 580.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_25193_full_validation.pdf.gz emd_25193_full_validation.pdf.gz | 579.7 KB | Display | |
| Data in XML |  emd_25193_validation.xml.gz emd_25193_validation.xml.gz | 6.7 KB | Display | |
| Data in CIF |  emd_25193_validation.cif.gz emd_25193_validation.cif.gz | 7.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25193 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25193 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25193 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-25193 | HTTPS FTP |
-Related structure data
| Related structure data |  7sl7MC  7sl1C  7sl2C  7sl3C  7sl4C  7sl6C  7sthC  7stiC  7stjC  7stkC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_25193.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_25193.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
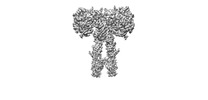
| Annotation | Cryo-EM structure of full-length insulin receptor bound with both site 1 binding deficient mutant insulin (A-V3E) and site 2 binding deficient mutant insulin (A-L13R) | ||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.83 Å | ||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : Full-length insulin receptor bound with both site 1 binding defic...
| Entire | Name: Full-length insulin receptor bound with both site 1 binding deficient mutant insulin (A-V3E) and site 2 binding deficient mutant insulin (A-L13R) |
|---|---|
| Components |
|
-Supramolecule #1: Full-length insulin receptor bound with both site 1 binding defic...
| Supramolecule | Name: Full-length insulin receptor bound with both site 1 binding deficient mutant insulin (A-V3E) and site 2 binding deficient mutant insulin (A-L13R) type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|
-Macromolecule #1: Insulin receptor
| Macromolecule | Name: Insulin receptor / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO / EC number: receptor protein-tyrosine kinase |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 155.790516 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MGFGRGCETT AVPLLVAVAA LLVGTAGHLY PGEVCPGMDI RNNLTRLHEL ENCSVIEGHL QILLMFKTRP EDFRDLSFPK LIMITDYLL LFRVYGLESL KDLFPNLTVI RGSRLFFNYA LVIFEMVHLK ELGLYNLMNI TRGSVRIEKN NELCYLATID W SRILDSVE ...String: MGFGRGCETT AVPLLVAVAA LLVGTAGHLY PGEVCPGMDI RNNLTRLHEL ENCSVIEGHL QILLMFKTRP EDFRDLSFPK LIMITDYLL LFRVYGLESL KDLFPNLTVI RGSRLFFNYA LVIFEMVHLK ELGLYNLMNI TRGSVRIEKN NELCYLATID W SRILDSVE DNYIVLNKDD NEECGDVCPG TAKGKTNCPA TVINGQFVER CWTHSHCQKV CPTICKSHGC TAEGLCCHKE CL GNCSEPD DPTKCVACRN FYLDGQCVET CPPPYYHFQD WRCVNFSFCQ DLHFKCRNSR KPGCHQYVIH NNKCIPECPS GYT MNSSNL MCTPCLGPCP KVCQILEGEK TIDSVTSAQE LRGCTVINGS LIINIRGGNN LAAELEANLG LIEEISGFLK IRRS YALVS LSFFRKLHLI RGETLEIGNY SFYALDNQNL RQLWDWSKHN LTITQGKLFF HYNPKLCLSE IHKMEEVSGT KGRQE RNDI ALKTNGDQAS CENELLKFSF IRTSFDKILL RWEPYWPPDF RDLLGFMLFY KEAPYQNVTE FDGQDACGSN SWTVVD IDP PQRSNDPKSQ TPSHPGWLMR GLKPWTQYAI FVKTLVTFSD ERRTYGAKSD IIYVQTDATN PSVPLDPISV SNSSSQI IL KWKPPSDPNG NITHYLVYWE RQAEDSELFE LDYCLKGLKL PSRTWSPPFE SDDSQKHNQS EYDDSASECC SCPKTDSQ I LKELEESSFR KTFEDYLHNV VFVPRPSRKR RSLEEVGNVT ATTLTLPDFP NVSSTIVPTS QEEHRPFEKV VNKESLVIS GLRHFTGYRI ELQACNQDSP DERCSVAAYV SARTMPEAKA DDIVGPVTHE IFENNVVHLM WQEPKEPNGL IVLYEVSYRR YGDEELHLC VSRKHFALER GCRLRGLSPG NYSVRVRATS LAGNGSWTEP TYFYVTDYLD VPSNIAKIII GPLIFVFLFS V VIGSIYLF LRKRQPDGPM GPLYASSNPE YLSASDVFPS SVYVPDEWEV PREKITLLRE LGQGSFGMVY EGNAKDIIKG EA ETRVAVK TVNESASLRE RIEFLNEASV MKGFTCHHVV RLLGVVSKGQ PTLVVMELMA HGDLKSHLRS LRPDAENNPG RPP PTLQEM IQMTAEIADG MAYLNAKKFV HRDLAARNCM VAHDFTVKIG DFGMTRDIYE TDYYRKGGKG LLPVRWMSPE SLKD GVFTA SSDMWSFGVV LWEITSLAEQ PYQGLSNEQV LKFVMDGGYL DPPDNCPERL TDLMRMCWQF NPKMRPTFLE IVNLL KDDL HPSFPEVSFF YSEENKAPES EELEMEFEDM ENVPLDRSSH CQREEAGGRE GGSSLSIKRT YDEHIPYTHM NGGKKN GRV LTLPRSNPS UniProtKB: Insulin receptor |
-Macromolecule #2: Insulin B chain
| Macromolecule | Name: Insulin B chain / type: protein_or_peptide / ID: 2 / Number of copies: 4 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 3.433953 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: FVNQHLCGSH LVEALYLVCG ERGFFYTPKT UniProtKB: Insulin |
-Macromolecule #3: Insulin A chain (L13R)
| Macromolecule | Name: Insulin A chain (L13R) / type: protein_or_peptide / ID: 3 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 2.427734 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: GIVEQCCTSI CSRYQLENYC N UniProtKB: Insulin |
-Macromolecule #4: Insulin A chain (V3E)
| Macromolecule | Name: Insulin A chain (V3E) / type: protein_or_peptide / ID: 4 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 2.413681 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: GIEEQCCTSI CSLYQLENYC N UniProtKB: Insulin |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 6 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.6 µm / Nominal defocus min: 1.6 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller


































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)




















