[English] 日本語
 Yorodumi
Yorodumi- EMDB-24471: Cre recombinase mutant (D33A/A36V/R192A) in complex with loxA DNA... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-24471 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cre recombinase mutant (D33A/A36V/R192A) in complex with loxA DNA hairpin | |||||||||
 Map data Map data | Structure of Cre recombinase mutant (D33A/A36V/R192A) in complex with a loxA DNA hairpin. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Cre / recombinase / monomer / hairpin / RECOMBINATION-DNA complex | |||||||||
| Function / homology |  Function and homology information Function and homology information | |||||||||
| Biological species |  Escherichia phage P1 (virus) Escherichia phage P1 (virus) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.91 Å | |||||||||
 Authors Authors | Stachowski K / Foster MP | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2022 Journal: Nucleic Acids Res / Year: 2022Title: Mechanisms of Cre recombinase synaptic complex assembly and activation illuminated by Cryo-EM. Authors: Kye Stachowski / Andrew S Norris / Devante Potter / Vicki H Wysocki / Mark P Foster /  Abstract: Cre recombinase selectively recognizes DNA and prevents non-specific DNA cleavage through an orchestrated series of assembly intermediates. Cre recombines two loxP DNA sequences featuring a pair of ...Cre recombinase selectively recognizes DNA and prevents non-specific DNA cleavage through an orchestrated series of assembly intermediates. Cre recombines two loxP DNA sequences featuring a pair of palindromic recombinase binding elements and an asymmetric spacer region, by assembly of a tetrameric synaptic complex, cleavage of an opposing pair of strands, and formation of a Holliday junction intermediate. We used Cre and loxP variants to isolate the monomeric Cre-loxP (54 kDa), dimeric Cre2-loxP (110 kDa), and tetrameric Cre4-loxP2 assembly intermediates, and determined their structures using cryo-EM to resolutions of 3.9, 4.5 and 3.2 Å, respectively. Progressive and asymmetric bending of the spacer region along the assembly pathway enables formation of increasingly intimate interfaces between Cre protomers and illuminates the structural bases of biased loxP strand cleavage order and half-the-sites activity. Application of 3D variability analysis to the tetramer data reveals constrained conformational sampling along the pathway between protomer activation and Holliday junction isomerization. These findings underscore the importance of protein and DNA flexibility in Cre-mediated site selection, controlled activation of alternating protomers, the basis for biased strand cleavage order, and recombination efficiency. Such considerations may advance development of site-specific recombinases for use in gene editing applications. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_24471.map.gz emd_24471.map.gz | 59.8 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-24471-v30.xml emd-24471-v30.xml emd-24471.xml emd-24471.xml | 20.2 KB 20.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_24471.png emd_24471.png | 46.3 KB | ||
| Masks |  emd_24471_msk_1.map emd_24471_msk_1.map | 64 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-24471.cif.gz emd-24471.cif.gz | 6.6 KB | ||
| Others |  emd_24471_half_map_1.map.gz emd_24471_half_map_1.map.gz emd_24471_half_map_2.map.gz emd_24471_half_map_2.map.gz | 59.3 MB 59.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-24471 http://ftp.pdbj.org/pub/emdb/structures/EMD-24471 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24471 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24471 | HTTPS FTP |
-Validation report
| Summary document |  emd_24471_validation.pdf.gz emd_24471_validation.pdf.gz | 765.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_24471_full_validation.pdf.gz emd_24471_full_validation.pdf.gz | 764.8 KB | Display | |
| Data in XML |  emd_24471_validation.xml.gz emd_24471_validation.xml.gz | 12.3 KB | Display | |
| Data in CIF |  emd_24471_validation.cif.gz emd_24471_validation.cif.gz | 14.3 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24471 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24471 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24471 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24471 | HTTPS FTP |
-Related structure data
| Related structure data |  7rhyMC  7rhxC  7rhzC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_24471.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_24471.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
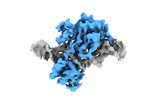
| Annotation | Structure of Cre recombinase mutant (D33A/A36V/R192A) in complex with a loxA DNA hairpin. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.95977 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
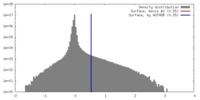
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_24471_msk_1.map emd_24471_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
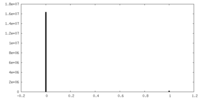
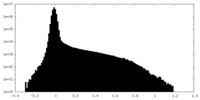
| Density Histograms |
-Half map: #2
| File | emd_24471_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
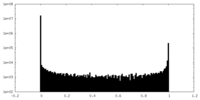
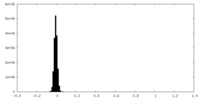
| Density Histograms |
-Half map: #1
| File | emd_24471_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
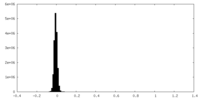
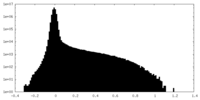
| Density Histograms |
- Sample components
Sample components
-Entire : Complex of Cre recombinase mutant D33A/A36V/R192A and loxA DNA hairpin
| Entire | Name: Complex of Cre recombinase mutant D33A/A36V/R192A and loxA DNA hairpin |
|---|---|
| Components |
|
-Supramolecule #1: Complex of Cre recombinase mutant D33A/A36V/R192A and loxA DNA hairpin
| Supramolecule | Name: Complex of Cre recombinase mutant D33A/A36V/R192A and loxA DNA hairpin type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Escherichia phage P1 (virus) Escherichia phage P1 (virus) |
| Molecular weight | Theoretical: 54 KDa |
-Macromolecule #1: Recombinase cre
| Macromolecule | Name: Recombinase cre / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Escherichia phage P1 (virus) Escherichia phage P1 (virus) |
| Molecular weight | Theoretical: 38.493094 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSNLLTVHQN LPALPVDATS DEVRKNLMDM FRARQVFSEH TWKMLLSVCR SWAAWCKLNN RKWFPAEPED VRDYLLYLQA RGLAVKTIQ QHLGQLNMLH RRSGLPRPSD SNAVSLVMRR IRKENVDAGE RAKQALAFER TDFDQVRSLM ENSDRCQDIR N LAFLGIAY ...String: MSNLLTVHQN LPALPVDATS DEVRKNLMDM FRARQVFSEH TWKMLLSVCR SWAAWCKLNN RKWFPAEPED VRDYLLYLQA RGLAVKTIQ QHLGQLNMLH RRSGLPRPSD SNAVSLVMRR IRKENVDAGE RAKQALAFER TDFDQVRSLM ENSDRCQDIR N LAFLGIAY NTLLRIAEIA RIRVKDISRT DGGAMLIHIG RTKTLVSTAG VEKALSLGVT KLVERWISVS GVADDPNNYL FC RVRKNGV AAPSATSQLS TRALEGIFEA THRLIYGAKD DSGQRYLAWS GHSARVGAAR DMARAGVSIP EIMQAGGWTN VNI VMNYIR NLDSETGAMV RLLEDGD UniProtKB: Recombinase cre |
-Macromolecule #2: DNA (49-MER)
| Macromolecule | Name: DNA (49-MER) / type: dna / ID: 2 / Number of copies: 1 / Classification: DNA |
|---|---|
| Source (natural) | Organism:  Escherichia phage P1 (virus) Escherichia phage P1 (virus) |
| Molecular weight | Theoretical: 15.119743 KDa |
| Sequence | String: (DG)(DC)(DA)(DT)(DA)(DA)(DC)(DT)(DT)(DC) (DG)(DT)(DA)(DT)(DA)(DG)(DC)(DA)(DT)(DA) (DT)(DG)(DC)(DG)(DA)(DA)(DG)(DC)(DA) (DT)(DA)(DT)(DG)(DC)(DT)(DA)(DT)(DA)(DC) (DG) (DA)(DA)(DG)(DT)(DT)(DA)(DT)(DG) (DC) |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 7.5 mg/mL | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7 Component:
Details: Buffer was made fresh from solid reagents and filtered with a 0.22 um filter. | |||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 60 sec. / Pretreatment - Atmosphere: AIR / Details: Pelco easiGLOW at 20 mA | |||||||||||||||
| Vitrification | Cryogen name: ETHANE-PROPANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Temperature | Min: 86.0 K / Max: 86.0 K |
| Specialist optics | Spherical aberration corrector: Microscope was modified with a Cs corrector with two hexapoles. Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 15 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Digitization - Dimensions - Width: 5760 pixel / Digitization - Dimensions - Height: 4096 pixel / Number grids imaged: 1 / Number real images: 6235 / Average exposure time: 2.0 sec. / Average electron dose: 60.0 e/Å2 / Details: 45 total frames |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 100.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model | PDB ID: Chain - Chain ID: A / Chain - Residue range: 20-343 / Chain - Source name: PDB / Chain - Initial model type: experimental model |
|---|---|
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Overall B value: 248.4 / Target criteria: correlation coefficient |
| Output model |  PDB-7rhy: |
 Movie
Movie Controller
Controller

















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)