[English] 日本語
 Yorodumi
Yorodumi- EMDB-24387: Cryo-EM structure of the unliganded form of NLR family apoptosis ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of the unliganded form of NLR family apoptosis inhibitory protein 5 (NAIP5) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | pre-liganded NAIP5 / inflammasome / innate immunity / host-pathogen interaction / IMMUNE SYSTEM | |||||||||
| Function / homology |  Function and homology information Function and homology informationIPAF inflammasome complex / cysteine-type endopeptidase inhibitor activity involved in apoptotic process / pyroptotic inflammatory response / detection of bacterium / positive regulation of interleukin-1 beta production / defense response to Gram-negative bacterium / defense response to bacterium / inflammatory response / symbiont entry into host cell / innate immune response ...IPAF inflammasome complex / cysteine-type endopeptidase inhibitor activity involved in apoptotic process / pyroptotic inflammatory response / detection of bacterium / positive regulation of interleukin-1 beta production / defense response to Gram-negative bacterium / defense response to bacterium / inflammatory response / symbiont entry into host cell / innate immune response / apoptotic process / negative regulation of apoptotic process / ATP binding / metal ion binding Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.3 Å | |||||||||
 Authors Authors | Paidimuddala B / Cao J / Xie Q / Wu H / Zhang L | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2023 Journal: Nat Struct Mol Biol / Year: 2023Title: Mechanism of NAIP-NLRC4 inflammasome activation revealed by cryo-EM structure of unliganded NAIP5. Authors: Bhaskar Paidimuddala / Jianhao Cao / Grady Nash / Qing Xie / Hao Wu / Liman Zhang /  Abstract: The nucleotide-binding domain (NBD), leucine rich repeat (LRR) domain containing protein family (NLR family) apoptosis inhibitory proteins (NAIPs) are cytosolic receptors that play critical roles in ...The nucleotide-binding domain (NBD), leucine rich repeat (LRR) domain containing protein family (NLR family) apoptosis inhibitory proteins (NAIPs) are cytosolic receptors that play critical roles in the host defense against bacterial infection. NAIPs interact with conserved bacterial ligands and activate the NLR family caspase recruitment domain containing protein 4 (NLRC4) to initiate the NAIP-NLRC4 inflammasome pathway. Here we found the process of NAIP activation is completely different from NLRC4. Our cryo-EM structure of unliganded mouse NAIP5 adopts an unprecedented wide-open conformation, with the nucleating surface fully exposed and accessible to recruit inactive NLRC4. Upon ligand binding, the winged helix domain (WHD) of NAIP5 undergoes roughly 20° rotation to form a steric clash with the inactive NLRC4, which triggers the conformational change of NLRC4 from inactive to active state. We also show the rotation of WHD places the 17-18 loop at a position that directly bind the active NLRC4 and stabilize the NAIP5-NLRC4 complex. Overall, these data provide structural mechanisms of inactive NAIP5, the process of NAIP5 activation and NAIP-dependent NLRC4 activation. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_24387.map.gz emd_24387.map.gz | 56.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-24387-v30.xml emd-24387-v30.xml emd-24387.xml emd-24387.xml | 16.6 KB 16.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_24387.png emd_24387.png | 95.4 KB | ||
| Filedesc metadata |  emd-24387.cif.gz emd-24387.cif.gz | 6.6 KB | ||
| Others |  emd_24387_half_map_1.map.gz emd_24387_half_map_1.map.gz emd_24387_half_map_2.map.gz emd_24387_half_map_2.map.gz | 59.3 MB 59.3 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-24387 http://ftp.pdbj.org/pub/emdb/structures/EMD-24387 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24387 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-24387 | HTTPS FTP |
-Validation report
| Summary document |  emd_24387_validation.pdf.gz emd_24387_validation.pdf.gz | 593.3 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_24387_full_validation.pdf.gz emd_24387_full_validation.pdf.gz | 592.9 KB | Display | |
| Data in XML |  emd_24387_validation.xml.gz emd_24387_validation.xml.gz | 12.2 KB | Display | |
| Data in CIF |  emd_24387_validation.cif.gz emd_24387_validation.cif.gz | 14.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24387 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24387 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24387 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-24387 | HTTPS FTP |
-Related structure data
| Related structure data |  7ravMC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_24387.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_24387.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.0694 Å | ||||||||||||||||||||||||||||||||||||
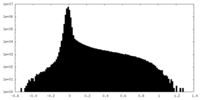
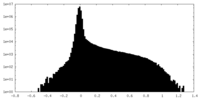
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_24387_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
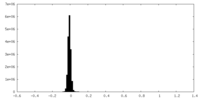
| Density Histograms |
-Half map: #1
| File | emd_24387_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
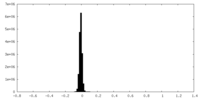
| Density Histograms |
- Sample components
Sample components
-Entire : pre-liganded form of NAIP5
| Entire | Name: pre-liganded form of NAIP5 |
|---|---|
| Components |
|
-Supramolecule #1: pre-liganded form of NAIP5
| Supramolecule | Name: pre-liganded form of NAIP5 / type: cell / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Baculoviral IAP repeat-containing protein 1e
| Macromolecule | Name: Baculoviral IAP repeat-containing protein 1e / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 161.128156 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: MDYKDDDDKL AEHGESSEDR ISEIDYEFLP ELSALLGVDA FQVAKSQEEE EHKERMKMKK GFNSQMRSEA KRLKTFETYD TFRSWTPQE MAAAGFYHTG VRLGVQCFCC SLILFGNSLR KLPIERHKKL RPECEFLQGK DVGNIGKYDI RVKRPEKMLR G GKARYHEE ...String: MDYKDDDDKL AEHGESSEDR ISEIDYEFLP ELSALLGVDA FQVAKSQEEE EHKERMKMKK GFNSQMRSEA KRLKTFETYD TFRSWTPQE MAAAGFYHTG VRLGVQCFCC SLILFGNSLR KLPIERHKKL RPECEFLQGK DVGNIGKYDI RVKRPEKMLR G GKARYHEE EARLESFEDW PFYAHGTSPR VLSAAGFVFT GKRDTVQCFS CGGSLGNWEE GDDPWKEHAK WFPKCEFLQS KK SSEEIAQ YIQSYEGFVH VTGEHFVKSW VRRELPMVSA YCNDSVFANE ELRMDMFKDW PQESPVGVEA LVRAGFFYTG KKD IVRCFS CGGCLEKWAE GDDPMEDHIK FFPECVFLQT LKSSAEVIPT LQSQYALPEA TETTRESNHG DAAAVHSTVV DLGR SEAQW FQEARSLSEQ LRDNYTKATF RHMNLPEVCS SLGTDHLLSC DVSIISKHIS QPVQEALTIP EVFSNLNSVM CVEGE TGSG KTTFLKRIAF LWASGCCPLL YRFQLVFYLS LSSITPDQGL ANIICAQLLG AGGCISEVCL SSSIQQLQHQ VLFLLD DYS GLASLPQALH TLITKNYLSR TCLLIAVHTN RVRDIRLYLG TSLEIQEFPF YNTVSVLRKF FSHDIICVEK LIIYFID NK DLQGVYKTPL FVAAVCTDWI QNASAQDKFQ DVTLFQSYMQ YLSLKYKATA EPLQATVSSC GQLALTGLFS SCFEFNSD D LAEAGVDEDE KLTTLLMSKF TAQRLRPVYR FLGPLFQEFL AAVRLTELLS SDRQEDQDLG LYYLRQIDSP LKAINSFNI FLYYVSSHSS SKAAPTVVSH LLQLVDEKES LENMSENEDY MKLHPQTFLW FQFVRGLWLV SPESSSSFVS EHLLRLALIF AYESNTVAE CSPFILQFLR GKTLALRVLN LQYFRDHPES LLLLRSLKVS INGNKMSSYV DYSFKTYFEN LQPPAIDEEY T SAFEHISE WRRNFAQDEE IIKNYENIRP RALPDISEGY WKLSPKPCKI PKLEVQVNNT DAADQALLQV LMEVFSASQS IE FRLFNSS GFLESICPAL ELSKASVTKC SMSRLELSRA EQELLLTLPA LQSLEVSETN QLPEQLFHNL HKFLGLKELC VRL DGKPNV LSVLPREFPN LLHMEKLSIQ TSTESDLSKL VKFIQNFPNL HVFHLKCDFL SNCESLMAVL ASCKKLREIE FSGR CFEAM TFVNILPNFV SLKILNLKDQ QFPDKETSEK FAQALGSLRN LEELLVPTGD GIHQVAKLIV RQCLQLPCLR VLTFH DILD DDSVIEIARA ATSGGFQKLE NLDISMNHKI TEEGYRNFFQ ALDNLPNLQE LNICRNIPGR IQVQATTVKA LGQCVS RLP SLIRLHMLSW LLDEEDMKVI NDVKERHPQS KRLIIFWKLI VPFSPVILE UniProtKB: Baculoviral IAP repeat-containing protein 1e |
-Macromolecule #2: ADENOSINE-5'-TRIPHOSPHATE
| Macromolecule | Name: ADENOSINE-5'-TRIPHOSPHATE / type: ligand / ID: 2 / Number of copies: 1 / Formula: ATP |
|---|---|
| Molecular weight | Theoretical: 507.181 Da |
| Chemical component information |  ChemComp-ATP: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | cell |
- Sample preparation
Sample preparation
| Concentration | 0.30 mg/mL |
|---|---|
| Buffer | pH: 8 |
| Grid | Model: UltrAuFoil R1.2/1.3 / Material: GOLD / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Energy filter - Name: GIF Bioquantum / Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 52.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 1.0 µm |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Final reconstruction | Applied symmetry - Point group: C1 (asymmetric) / Resolution.type: BY AUTHOR / Resolution: 3.3 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: cryoSPARC / Number images used: 159513 |
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
-Atomic model buiding 1
| Initial model |
| ||||||
|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: BACKBONE TRACE | ||||||
| Output model |  PDB-7rav: |
 Movie
Movie Controller
Controller




 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)