[English] 日本語
 Yorodumi
Yorodumi- EMDB-23655: Asymmetric Activation of the Calcium Sensing Receptor Homodimer -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23655 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Asymmetric Activation of the Calcium Sensing Receptor Homodimer | |||||||||
 Map data Map data | inactive-state human calcium sensing receptor complexed with NPS-2143, composite map of ECD and TMD local refinement maps. | |||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationbile acid secretion / chemosensory behavior / response to fibroblast growth factor / cellular response to peptide / cellular response to vitamin D / phosphatidylinositol phospholipase C activity / Class C/3 (Metabotropic glutamate/pheromone receptors) / calcium ion import / positive regulation of positive chemotaxis / cellular response to low-density lipoprotein particle stimulus ...bile acid secretion / chemosensory behavior / response to fibroblast growth factor / cellular response to peptide / cellular response to vitamin D / phosphatidylinositol phospholipase C activity / Class C/3 (Metabotropic glutamate/pheromone receptors) / calcium ion import / positive regulation of positive chemotaxis / cellular response to low-density lipoprotein particle stimulus / fat pad development / cellular response to hepatocyte growth factor stimulus / amino acid binding / branching morphogenesis of an epithelial tube / positive regulation of calcium ion import / regulation of calcium ion transport / positive regulation of vasoconstriction / detection of calcium ion / anatomical structure morphogenesis / ossification / axon terminus / JNK cascade / chloride transmembrane transport / adenylate cyclase-inhibiting G protein-coupled receptor signaling pathway / response to ischemia / G protein-coupled receptor activity / cellular response to glucose stimulus / vasodilation / positive regulation of insulin secretion / intracellular calcium ion homeostasis / integrin binding / cellular response to hypoxia / phospholipase C-activating G protein-coupled receptor signaling pathway / basolateral plasma membrane / G alpha (i) signalling events / G alpha (q) signalling events / transmembrane transporter binding / positive regulation of ERK1 and ERK2 cascade / apical plasma membrane / G protein-coupled receptor signaling pathway / neuronal cell body / positive regulation of cell population proliferation / calcium ion binding / positive regulation of gene expression / protein kinase binding / cell surface / protein homodimerization activity / identical protein binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 4.1 Å | |||||||||
 Authors Authors | Gao Y / Robertson MJ / Zhang C / Meyerowitz JG / Panova O / Skiniotis G | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2021 Journal: Nature / Year: 2021Title: Asymmetric activation of the calcium-sensing receptor homodimer. Authors: Yang Gao / Michael J Robertson / Sabrina N Rahman / Alpay B Seven / Chensong Zhang / Justin G Meyerowitz / Ouliana Panova / Fadil M Hannan / Rajesh V Thakker / Hans Bräuner-Osborne / Jesper ...Authors: Yang Gao / Michael J Robertson / Sabrina N Rahman / Alpay B Seven / Chensong Zhang / Justin G Meyerowitz / Ouliana Panova / Fadil M Hannan / Rajesh V Thakker / Hans Bräuner-Osborne / Jesper M Mathiesen / Georgios Skiniotis /    Abstract: The calcium-sensing receptor (CaSR), a cell-surface sensor for Ca, is the master regulator of calcium homeostasis in humans and is the target of calcimimetic drugs for the treatment of parathyroid ...The calcium-sensing receptor (CaSR), a cell-surface sensor for Ca, is the master regulator of calcium homeostasis in humans and is the target of calcimimetic drugs for the treatment of parathyroid disorders. CaSR is a family C G-protein-coupled receptor that functions as an obligate homodimer, with each protomer composed of a Ca-binding extracellular domain and a seven-transmembrane-helix domain (7TM) that activates heterotrimeric G proteins. Here we present cryo-electron microscopy structures of near-full-length human CaSR in inactive or active states bound to Ca and various calcilytic or calcimimetic drug molecules. We show that, upon activation, the CaSR homodimer adopts an asymmetric 7TM configuration that primes one protomer for G-protein coupling. This asymmetry is stabilized by 7TM-targeting calcimimetic drugs adopting distinctly different poses in the two protomers, whereas the binding of a calcilytic drug locks CaSR 7TMs in an inactive symmetric configuration. These results provide a detailed structural framework for CaSR activation and the rational design of therapeutics targeting this receptor. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23655.map.gz emd_23655.map.gz | 1.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23655-v30.xml emd-23655-v30.xml emd-23655.xml emd-23655.xml | 19 KB 19 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_23655.png emd_23655.png | 117.5 KB | ||
| Others |  emd_23655_additional_1.map.gz emd_23655_additional_1.map.gz emd_23655_additional_2.map.gz emd_23655_additional_2.map.gz emd_23655_additional_3.map.gz emd_23655_additional_3.map.gz | 168.1 MB 7.9 MB 171.5 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23655 http://ftp.pdbj.org/pub/emdb/structures/EMD-23655 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23655 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23655 | HTTPS FTP |
-Related structure data
| Related structure data |  7m3jMC  7m3eC  7m3fC  7m3gC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_23655.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23655.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | inactive-state human calcium sensing receptor complexed with NPS-2143, composite map of ECD and TMD local refinement maps. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.11 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
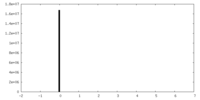
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Additional map: inactive-state human calcium sensing receptor complexed with NPS-2143,...
| File | emd_23655_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | inactive-state human calcium sensing receptor complexed with NPS-2143, global map without local refinement. | ||||||||||||
| Projections & Slices |
| ||||||||||||
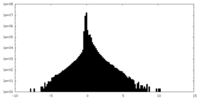
| Density Histograms |
-Additional map: inactive-state human calcium sensing receptor complexed with NPS-2143,...
| File | emd_23655_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | inactive-state human calcium sensing receptor complexed with NPS-2143, TMD local refinement map. | ||||||||||||
| Projections & Slices |
| ||||||||||||
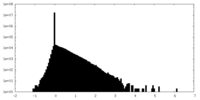
| Density Histograms |
-Additional map: inactive-state human calcium sensing receptor complexed with NPS-2143,...
| File | emd_23655_additional_3.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | inactive-state human calcium sensing receptor complexed with NPS-2143, ECD local refinement map. | ||||||||||||
| Projections & Slices |
| ||||||||||||
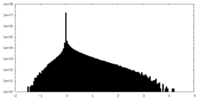
| Density Histograms |
- Sample components
Sample components
-Entire : inactive-state human extracellular calcium-sensing receptor compl...
| Entire | Name: inactive-state human extracellular calcium-sensing receptor complexed with negative allosteric modulator NPS-2143 |
|---|---|
| Components |
|
-Supramolecule #1: inactive-state human extracellular calcium-sensing receptor compl...
| Supramolecule | Name: inactive-state human extracellular calcium-sensing receptor complexed with negative allosteric modulator NPS-2143 type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Recombinant expression | Organism:  |
| Molecular weight | Theoretical: 200 kDa/nm |
-Macromolecule #1: Extracellular calcium-sensing receptor
| Macromolecule | Name: Extracellular calcium-sensing receptor / type: protein_or_peptide / ID: 1 Details: 1-16 is signaling sequence, 17-24 is FLAG epitope tag, 25-27 is an 3-alanine linker, the receptor sequence starts at residue Y28 that should be re-numbered to 20, and ends at V902 that ...Details: 1-16 is signaling sequence, 17-24 is FLAG epitope tag, 25-27 is an 3-alanine linker, the receptor sequence starts at residue Y28 that should be re-numbered to 20, and ends at V902 that should be re-numbered to 894. Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 101.745445 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MKTIIALSYI FCLVFADYKD DDDKAAAYGP DQRAQKKGDI ILGGLFPIHF GVAAKDQDLK SRPESVECIR YNFRGFRWLQ AMIFAIEEI NSSPALLPNL TLGYRIFDTC NTVSKALEAT LSFVAQNKID SLNLDEFCNC SEHIPSTIAV VGATGSGVST A VANLLGLF ...String: MKTIIALSYI FCLVFADYKD DDDKAAAYGP DQRAQKKGDI ILGGLFPIHF GVAAKDQDLK SRPESVECIR YNFRGFRWLQ AMIFAIEEI NSSPALLPNL TLGYRIFDTC NTVSKALEAT LSFVAQNKID SLNLDEFCNC SEHIPSTIAV VGATGSGVST A VANLLGLF YIPQVSYASS SRLLSNKNQF KSFLRTIPND EHQATAMADI IEYFRWNWVG TIAADDDYGR PGIEKFREEA EE RDICIDF SELISQYSDE EEIQHVVEVI QNSTAKVIVV FSSGPDLEPL IKEIVRRNIT GKIWLASEAW ASSSLIAMPQ YFH VVGGTI GFALKAGQIP GFREFLKKVH PRKSVHNGFA KEFWEETFNC HLQEGAKGPL PVDTFLRGHE ESGDRFSNSS TAFR PLCTG DENISSVETP YIDYTHLRIS YNVYLAVYSI AHALQDIYTC LPGRGLFTNG SCADIKKVEA WQVLKHLRHL NFTNN MGEQ VTFDECGDLV GNYSIINWHL SPEDGSIVFK EVGYYNVYAK KGERLFINEE KILWSGFSRE VPFSNCSRDC LAGTRK GII EGEPTCCFEC VECPDGEYSD ETDASACNKC PDDFWSNENH TSCIAKEIEF LSWTEPFGIA LTLFAVLGIF LTAFVLG VF IKFRNTPIVK ATNRELSYLL LFSLLCCFSS SLFFIGEPQD WTCRLRQPAF GISFVLCISC ILVKTNRVLL VFEAKIPT S FHRKWWGLNL QFLLVFLCTF MQIVICVIWL YTAPPSSYRN QELEDEIIFI TCHEGSLMAL GFLIGYTCLL AAICFFFAF KSRKLPENFN EAKFITFSML IFFIVWISFI PAYASTYGKF VSAVEVIAIL AASFGLLACI FFNKIYIILF KPSRNTIEEV RCSTAAHAF KVAARATLRR SNV |
-Macromolecule #3: 2-chloro-6-[(2R)-2-hydroxy-3-{[2-methyl-1-(naphthalen-2-yl)propan...
| Macromolecule | Name: 2-chloro-6-[(2R)-2-hydroxy-3-{[2-methyl-1-(naphthalen-2-yl)propan-2-yl]amino}propoxy]benzonitrile type: ligand / ID: 3 / Number of copies: 2 / Formula: YP1 |
|---|---|
| Molecular weight | Theoretical: 408.921 Da |
| Chemical component information |  ChemComp-YP1: |
-Macromolecule #4: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 4 / Number of copies: 5 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #5: PHOSPHATE ION
| Macromolecule | Name: PHOSPHATE ION / type: ligand / ID: 5 / Number of copies: 2 / Formula: PO4 |
|---|---|
| Molecular weight | Theoretical: 94.971 Da |
| Chemical component information |  ChemComp-PO4: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 7 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 60.9 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 70.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 4.1 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 329093 |
|---|---|
| Initial angle assignment | Type: MAXIMUM LIKELIHOOD |
| Final angle assignment | Type: MAXIMUM LIKELIHOOD |
 Movie
Movie Controller
Controller














 Z
Z Y
Y X
X