[English] 日本語
 Yorodumi
Yorodumi- EMDB-23412: Cryo-EM map of BG505 DS-SOSIP in complex with Glycan276-Dependent... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23412 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM map of BG505 DS-SOSIP in complex with Glycan276-Dependent Broadly Neutralizing Antibody VRC33.01 Fab | |||||||||
 Map data Map data | Cryo-EM half map A of BG505 DS-SOSIP in complex with Glycan276-Dependent Broadly Neutralizing Antibodiy VRC33.01 Fab | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | BG505 DS-SOSIP / Glycan276-Dependent / Glycan276 / VRC33.01 Fab / HIV-1 / IMMUNE SYSTEM / IMMUNE SYSTEM-VIRAL PROTEIN complex | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / positive regulation of establishment of T cell polarity / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell / viral protein processing / fusion of virus membrane with host plasma membrane / virus-mediated perturbation of host defense response / fusion of virus membrane with host endosome membrane / viral envelope ...positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / positive regulation of establishment of T cell polarity / host cell endosome membrane / clathrin-dependent endocytosis of virus by host cell / viral protein processing / fusion of virus membrane with host plasma membrane / virus-mediated perturbation of host defense response / fusion of virus membrane with host endosome membrane / viral envelope / virion attachment to host cell / apoptotic process / host cell plasma membrane / structural molecule activity / virion membrane / identical protein binding / plasma membrane Similarity search - Function | |||||||||
| Biological species |   Human immunodeficiency virus 1 / Human immunodeficiency virus 1 /  Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.73 Å | |||||||||
 Authors Authors | Manne K / Acharya P | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Cell Rep / Year: 2021 Journal: Cell Rep / Year: 2021Title: Structural basis of glycan276-dependent recognition by HIV-1 broadly neutralizing antibodies. Authors: Christopher A Cottrell / Kartik Manne / Rui Kong / Shuishu Wang / Tongqing Zhou / Gwo-Yu Chuang / Robert J Edwards / Rory Henderson / Katarzyna Janowska / Megan Kopp / Bob C Lin / Mark K ...Authors: Christopher A Cottrell / Kartik Manne / Rui Kong / Shuishu Wang / Tongqing Zhou / Gwo-Yu Chuang / Robert J Edwards / Rory Henderson / Katarzyna Janowska / Megan Kopp / Bob C Lin / Mark K Louder / Adam S Olia / Reda Rawi / Chen-Hsiang Shen / Justin D Taft / Jonathan L Torres / Nelson R Wu / Baoshan Zhang / Nicole A Doria-Rose / Myron S Cohen / Barton F Haynes / Lawrence Shapiro / Andrew B Ward / Priyamvada Acharya / John R Mascola / Peter D Kwong /  Abstract: Recognition of N-linked glycan at residue N276 (glycan276) at the periphery of the CD4-binding site (CD4bs) on the HIV-envelope trimer is a formidable challenge for many CD4bs-directed antibodies. To ...Recognition of N-linked glycan at residue N276 (glycan276) at the periphery of the CD4-binding site (CD4bs) on the HIV-envelope trimer is a formidable challenge for many CD4bs-directed antibodies. To understand how this glycan can be recognized, here we isolate two lineages of glycan276-dependent CD4bs antibodies. Antibody CH540-VRC40.01 (named for donor-lineage.clone) neutralizes 81% of a panel of 208 diverse strains, while antibody CH314-VRC33.01 neutralizes 45%. Cryo-electron microscopy (cryo-EM) structures of these two antibodies and 179NC75, a previously identified glycan276-dependent CD4bs antibody, in complex with HIV-envelope trimer reveal substantially different modes of glycan276 recognition. Despite these differences, binding of glycan276-dependent antibodies maintains a glycan276 conformation similar to that observed in the absence of glycan276-binding antibodies. By contrast, glycan276-independent CD4bs antibodies, such as VRC01, displace glycan276 upon binding. These results provide a foundation for understanding antibody recognition of glycan276 and suggest its presence may be crucial for priming immunogens seeking to initiate broad CD4bs recognition. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23412.map.gz emd_23412.map.gz | 117.3 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23412-v30.xml emd-23412-v30.xml emd-23412.xml emd-23412.xml | 22.1 KB 22.1 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_23412.png emd_23412.png | 75.8 KB | ||
| Filedesc metadata |  emd-23412.cif.gz emd-23412.cif.gz | 7 KB | ||
| Others |  emd_23412_half_map_1.map.gz emd_23412_half_map_1.map.gz emd_23412_half_map_2.map.gz emd_23412_half_map_2.map.gz | 115.7 MB 115.7 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23412 http://ftp.pdbj.org/pub/emdb/structures/EMD-23412 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23412 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23412 | HTTPS FTP |
-Validation report
| Summary document |  emd_23412_validation.pdf.gz emd_23412_validation.pdf.gz | 760.8 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_23412_full_validation.pdf.gz emd_23412_full_validation.pdf.gz | 760.3 KB | Display | |
| Data in XML |  emd_23412_validation.xml.gz emd_23412_validation.xml.gz | 14.1 KB | Display | |
| Data in CIF |  emd_23412_validation.cif.gz emd_23412_validation.cif.gz | 16.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23412 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23412 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23412 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23412 | HTTPS FTP |
-Related structure data
| Related structure data |  7ll2MC  7lg6C  7ll1C  7llkC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_23412.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23412.map.gz / Format: CCP4 / Size: 125 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM half map A of BG505 DS-SOSIP in complex with Glycan276-Dependent Broadly Neutralizing Antibodiy VRC33.01 Fab | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.08 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Half map: Cryo-EM structure of BG505 DS-SOSIP in complex with...
| File | emd_23412_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM structure of BG505 DS-SOSIP in complex with Glycan276-Dependent Broadly Neutralizing Antibodiy VRC33.01 Fab | ||||||||||||
| Projections & Slices |
| ||||||||||||
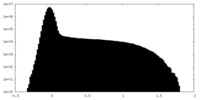
| Density Histograms |
-Half map: Cryo-EM half map B of BG505 DS-SOSIP in...
| File | emd_23412_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-EM half map B of BG505 DS-SOSIP in complex with Glycan276-Dependent Broadly Neutralizing Antibodiy VRC33.01 Fab | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Trimeric HIV-1 Env in complex with VRC33.01 Fab
| Entire | Name: Trimeric HIV-1 Env in complex with VRC33.01 Fab |
|---|---|
| Components |
|
-Supramolecule #1: Trimeric HIV-1 Env in complex with VRC33.01 Fab
| Supramolecule | Name: Trimeric HIV-1 Env in complex with VRC33.01 Fab / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
-Macromolecule #1: Envelope glycoprotein gp120
| Macromolecule | Name: Envelope glycoprotein gp120 / type: protein_or_peptide / ID: 1 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
| Molecular weight | Theoretical: 52.986969 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: AENLWVTVYY GVPVWKDAET TLFCASDAKA YETEKHNVWA THACVPTDPN PQEIHLENVT EEFNMWKNNM VEQMHTDIIS LWDQSLKPC VKLTPLCVTL QCTNVTNNIT DDMRGELKNC SFNMTTELRD KKQKVYSLFY RLDVVQINEN QGNRSNNSNK E YRLINCNT ...String: AENLWVTVYY GVPVWKDAET TLFCASDAKA YETEKHNVWA THACVPTDPN PQEIHLENVT EEFNMWKNNM VEQMHTDIIS LWDQSLKPC VKLTPLCVTL QCTNVTNNIT DDMRGELKNC SFNMTTELRD KKQKVYSLFY RLDVVQINEN QGNRSNNSNK E YRLINCNT SACTQACPKV SFEPIPIHYC APAGFAILKC KDKKFNGTGP CPSVSTVQCT HGIKPVVSTQ LLLNGSLAEE EV MIRSENI TNNAKNILVQ FNTPVQINCT RPNNNTRKSI RIGPGQAFYA TGDIIGDIRQ AHCNVSKATW NETLGKVVKQ LRK HFGNNT IIRFANSSGG DLEVTTHSFN CGGEFFYCNT SGLFNSTWIS NTSVQGSNST GSNDSITLPC RIKQIINMWQ RIGQ CMYAP PIQGVIRCVS NITGLILTRD GGSTNSTTET FRPGGGDMRD NWRSELYKYK VVKIEPLGVA PTRCKRRV UniProtKB: Envelope glycoprotein gp160 |
-Macromolecule #2: Envelope glycoprotein gp41
| Macromolecule | Name: Envelope glycoprotein gp41 / type: protein_or_peptide / ID: 2 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Human immunodeficiency virus 1 Human immunodeficiency virus 1 |
| Molecular weight | Theoretical: 17.162525 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: AVGIGAVFLG FLGAAGSTMG AASMTLTVQA RNLLSGIVQQ QSNLLRAIEA QQHLLKLTVW GIKQLQARVL AVERYLRDQQ LLGIWGCSG KLICCTNVPW NSSWSNRNLS EIWDNMTWLQ WDKEISNYTQ IIYGLLEESQ NQQEKNEQDL LALD UniProtKB: Envelope glycoprotein gp160 |
-Macromolecule #3: VRC33.01 Fab Heavy chain
| Macromolecule | Name: VRC33.01 Fab Heavy chain / type: protein_or_peptide / ID: 3 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 24.030932 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: QVQLQQWGAG LLKPSETLSL TCALYGRSLN GNYWSWIRQS PGKGLEWIGE INHSGSTYFN PSFKSRVAMS VDTSKSQFSL KLNSVTAAD TGIYFCARGK RYSASYSNYF GVWGQGTQVT VSSASTKGPS VFPLAPSSKS TSGGTAALGC LVKDYFPEPV T VSWNSGAL ...String: QVQLQQWGAG LLKPSETLSL TCALYGRSLN GNYWSWIRQS PGKGLEWIGE INHSGSTYFN PSFKSRVAMS VDTSKSQFSL KLNSVTAAD TGIYFCARGK RYSASYSNYF GVWGQGTQVT VSSASTKGPS VFPLAPSSKS TSGGTAALGC LVKDYFPEPV T VSWNSGAL TSGVHTFPAV LQSSGLYSLS SVVTVPSSSL GTQTYICNVN HKPSNTKVDK KVEPKSC |
-Macromolecule #4: VRC33.01 Fab Light chain
| Macromolecule | Name: VRC33.01 Fab Light chain / type: protein_or_peptide / ID: 4 / Number of copies: 3 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 23.977701 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: DIQMTQSPST LSASVGDRVD ITCRASQSIS RWLAWYQQKP GKAPKVLIYE ASLLANGVPS RFSGHFNGRE SATDFTLTIS SLQPDDVAT YYCQHYMADP RFGQGTKLEI KRTVAAPSVF IFPPSDEQLK SGTASVVCLL NNFYPREAKV QWKVDNALQS G NSQESVTE ...String: DIQMTQSPST LSASVGDRVD ITCRASQSIS RWLAWYQQKP GKAPKVLIYE ASLLANGVPS RFSGHFNGRE SATDFTLTIS SLQPDDVAT YYCQHYMADP RFGQGTKLEI KRTVAAPSVF IFPPSDEQLK SGTASVVCLL NNFYPREAKV QWKVDNALQS G NSQESVTE QDSKDSTYSL SSTLTLSKAD YEKHKVYACE VTHQGLSSPV TKSFNRGEC |
-Macromolecule #9: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 9 / Number of copies: 12 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Grid | Model: Quantifoil R1.2/1.3 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 15 sec. / Pretreatment - Atmosphere: AIR / Pretreatment - Pressure: 0.039 kPa |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 95 % / Chamber temperature: 293 K / Instrument: LEICA EM GP / Details: Blot for 2.5 S before plunging. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 93.15 K / Max: 93.15 K |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 58.5 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||
|---|---|---|---|---|---|---|---|
| Refinement | Protocol: AB INITIO MODEL | ||||||
| Output model |  PDB-7ll2: |
 Movie
Movie Controller
Controller















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)