[English] 日本語
 Yorodumi
Yorodumi- EMDB-23060: The stress-sensing domain of activated IRE1a forms helical filame... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-23060 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
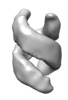
| Title | The stress-sensing domain of activated IRE1a forms helical filaments in narrow ER membrane tubes | ||||||||||||
 Map data Map data | Cryo-CLEM/cryo-ET was used to target IRE1a fused to fluorescent protein mNeonGreen (mNG) in U2OS cells. Helical symmetry applied using sym=H2.5:2:45:5 with --keep=0.9 --maxalt=50.0 --mask=Auto | ||||||||||||
 Sample Sample |
| ||||||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | ||||||||||||
| Method | subtomogram averaging / cryo EM / Resolution: 30.0 Å | ||||||||||||
 Authors Authors | Carter SD / Tran NH / De Maziere A / Ashkenazi A / Klumpermann J / Walter P / Jensen GJ | ||||||||||||
| Funding support |  United States, 3 items United States, 3 items
| ||||||||||||
 Citation Citation |  Journal: Science / Year: 2021 Journal: Science / Year: 2021Title: The stress-sensing domain of activated IRE1α forms helical filaments in narrow ER membrane tubes. Authors: Ngoc-Han Tran / Stephen D Carter / Ann De Mazière / Avi Ashkenazi / Judith Klumperman / Peter Walter / Grant J Jensen /   Abstract: The signaling network of the unfolded protein response (UPR) adjusts the protein-folding capacity of the endoplasmic reticulum (ER) according to need. The most conserved UPR sensor, IRE1α, spans the ...The signaling network of the unfolded protein response (UPR) adjusts the protein-folding capacity of the endoplasmic reticulum (ER) according to need. The most conserved UPR sensor, IRE1α, spans the ER membrane and activates through oligomerization. IRE1α oligomers accumulate in dynamic foci. We determined the in situ structure of IRE1α foci by cryogenic correlated light and electron microscopy combined with electron cryo-tomography and complementary immuno–electron microscopy in mammalian cell lines. IRE1α foci localized to a network of narrow anastomosing ER tubes (diameter, ~28 nm) with complex branching. The lumen of the tubes contained protein filaments, which were likely composed of arrays of IRE1α lumenal domain dimers that were arranged in two intertwined, left-handed helices. This specialized ER subdomain may play a role in modulating IRE1α signaling. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_23060.map.gz emd_23060.map.gz | 652.2 KB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-23060-v30.xml emd-23060-v30.xml emd-23060.xml emd-23060.xml | 12.9 KB 12.9 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_23060.png emd_23060.png | 32 KB | ||
| Masks |  emd_23060_msk_1.map emd_23060_msk_1.map | 687 KB |  Mask map Mask map | |
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-23060 http://ftp.pdbj.org/pub/emdb/structures/EMD-23060 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23060 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-23060 | HTTPS FTP |
-Validation report
| Summary document |  emd_23060_validation.pdf.gz emd_23060_validation.pdf.gz | 340.1 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_23060_full_validation.pdf.gz emd_23060_full_validation.pdf.gz | 339.7 KB | Display | |
| Data in XML |  emd_23060_validation.xml.gz emd_23060_validation.xml.gz | 4.6 KB | Display | |
| Data in CIF |  emd_23060_validation.cif.gz emd_23060_validation.cif.gz | 5.2 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23060 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23060 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23060 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-23060 | HTTPS FTP |
-Related structure data
| Related structure data | C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_23060.map.gz / Format: CCP4 / Size: 686.5 KB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_23060.map.gz / Format: CCP4 / Size: 686.5 KB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Cryo-CLEM/cryo-ET was used to target IRE1a fused to fluorescent protein mNeonGreen (mNG) in U2OS cells. Helical symmetry applied using sym=H2.5:2:45:5 with --keep=0.9 --maxalt=50.0 --mask=Auto | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 6.52 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_23060_msk_1.map emd_23060_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Double-helical arrangement of human IRE1a luminal domain in 30 nm...
| Entire | Name: Double-helical arrangement of human IRE1a luminal domain in 30 nm ER tubes |
|---|---|
| Components |
|
-Supramolecule #1: Double-helical arrangement of human IRE1a luminal domain in 30 nm...
| Supramolecule | Name: Double-helical arrangement of human IRE1a luminal domain in 30 nm ER tubes type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: all Details: Cryo-CLEM was used to target IRE1a fused to fluorescent protein mNeonGreen (mNG) for cryo-ET imaging. |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) / Organelle: ER Homo sapiens (human) / Organelle: ER |
-Macromolecule #1: IRE1a lumenal domain
| Macromolecule | Name: IRE1a lumenal domain / type: protein_or_peptide / ID: 1 / Enantiomer: LEVO |
|---|---|
| Sequence | String: MPARRLLLLL TLLLPGLGIF GSTSTVTLPE TLLFVSTLDG SLHAVSKRTG SIKWTLKEDP VLQVPTHVEE PAFLPDPNDG SLYTLGSKNN EGLTKLPFTI PELVQASPCR SSDGILYMGK KQDIWYVIDL LTGEKQQTLS SAFADSLCPS TSLLYLGRTE YTITMYDTKT ...String: MPARRLLLLL TLLLPGLGIF GSTSTVTLPE TLLFVSTLDG SLHAVSKRTG SIKWTLKEDP VLQVPTHVEE PAFLPDPNDG SLYTLGSKNN EGLTKLPFTI PELVQASPCR SSDGILYMGK KQDIWYVIDL LTGEKQQTLS SAFADSLCPS TSLLYLGRTE YTITMYDTKT RELRWNATYF DYAASLPEDD VDYKMSHFVS NGDGLVVTVD SESGDVLWIQ NYASPVVAFY VWQREGLRKV MHINVAVETL RYLTFMSGEV GRITKWKYPF PKETEAKSKL TPTLYVGKYS TSLYASPSMV HEGVAVVPRG STLPLLEGPQ TDGVTIGDKG ECVITPSTDV KFDPGLKSKN KLNYLRNYWL LIGHHETPLS ASTKMLERFP NNLPKHRENV IPADSEKKSF EEVINLVDQT SENAPTTVSR DVEEKPAHAP ARPEAPVDSM LKD |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | subtomogram averaging |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Buffer | pH: 7 |
|---|---|
| Grid | Model: Quantifoil R2/2 / Material: GOLD / Mesh: 200 / Support film - Material: CARBON / Support film - topology: HOLEY / Support film - Film thickness: 5.0 nm |
| Vitrification | Cryogen name: ETHANE-PROPANE / Chamber humidity: 90 % / Chamber temperature: 310.15 K / Instrument: FEI VITROBOT MARK III Details: Manual blotting from the gold side of the grid (opposite side to cells).. |
| Details | in situ imaging in a near-native state |
- Electron microscopy
Electron microscopy
| Microscope | FEI POLARA 300 |
|---|---|
| Specialist optics | Energy filter - Slit width: 20 eV |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 1.5 e/Å2 Details: Images were collected using a bidirectional tilt-scheme. |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.2 mm / Nominal magnification: 34000 |
| Sample stage | Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
- Image processing
Image processing
| Final reconstruction | Applied symmetry - Helical parameters - Δz: 32.6 Å Applied symmetry - Helical parameters - Δ&Phi: 45 ° Applied symmetry - Helical parameters - Axial symmetry: C2 (2 fold cyclic) Resolution.type: BY AUTHOR / Resolution: 30.0 Å / Resolution method: FSC 0.143 CUT-OFF / Software - Name: EMAN2 (ver. 2.3) / Number subtomograms used: 653 |
|---|---|
| Extraction | Number tomograms: 3 / Number images used: 653 / Method: Manually every ~85A along the filament / Details: e2boxer.py |
| CTF correction | Software - Name: EMAN2 (ver. 2.3) / Software - details: CTF / Details: CTF correction was performed in EMAN2 |
| Final angle assignment | Type: NOT APPLICABLE |
 Movie
Movie Controller
Controller





 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)