+ Open data
Open data
- Basic information
Basic information
| Entry |  | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Proteasomal late precursor complex from pre1-1 | ||||||||||||
 Map data Map data | |||||||||||||
 Sample Sample |
| ||||||||||||
 Keywords Keywords | proteasome biogenesis / Ump1 / pre1-1 / cryo-EM / propertied maturation / HYDROLASE | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of proteasome core complex assembly / proteasome core complex assembly / nuclear outer membrane-endoplasmic reticulum membrane network / Cross-presentation of soluble exogenous antigens (endosomes) / TNFR2 non-canonical NF-kB pathway / proteasomal ubiquitin-independent protein catabolic process / Ubiquitin Mediated Degradation of Phosphorylated Cdc25A / Regulation of PTEN stability and activity / KEAP1-NFE2L2 pathway / CDK-mediated phosphorylation and removal of Cdc6 ...regulation of proteasome core complex assembly / proteasome core complex assembly / nuclear outer membrane-endoplasmic reticulum membrane network / Cross-presentation of soluble exogenous antigens (endosomes) / TNFR2 non-canonical NF-kB pathway / proteasomal ubiquitin-independent protein catabolic process / Ubiquitin Mediated Degradation of Phosphorylated Cdc25A / Regulation of PTEN stability and activity / KEAP1-NFE2L2 pathway / CDK-mediated phosphorylation and removal of Cdc6 / Neddylation / FBXL7 down-regulates AURKA during mitotic entry and in early mitosis / Orc1 removal from chromatin / MAPK6/MAPK4 signaling / proteasome storage granule / Antigen processing: Ubiquitination & Proteasome degradation / endopeptidase activator activity / proteasome assembly / proteasome endopeptidase complex / proteasome core complex, beta-subunit complex / Ub-specific processing proteases / proteasome core complex, alpha-subunit complex / threonine-type endopeptidase activity / Neutrophil degranulation / proteasome complex / peroxisome / ubiquitin-dependent protein catabolic process / proteasome-mediated ubiquitin-dependent protein catabolic process / endopeptidase activity / mRNA binding / DNA damage response / endoplasmic reticulum membrane / mitochondrion / nucleus / cytosol / cytoplasm Similarity search - Function | ||||||||||||
| Biological species |  | ||||||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.14 Å | ||||||||||||
 Authors Authors | Mark E / Ramos PC / Kayser F / Hoeckendorff J / Dohmen RJ / Wendler P | ||||||||||||
| Funding support |  Germany, European Union, 3 items Germany, European Union, 3 items
| ||||||||||||
 Citation Citation |  Journal: Life Sci Alliance / Year: 2024 Journal: Life Sci Alliance / Year: 2024Title: Structural roles of Ump1 and β-subunit propeptides in proteasome biogenesis. Authors: Eric Mark / Paula C Ramos / Fleur Kayser / Jörg Höckendorff / R Jürgen Dohmen / Petra Wendler /  Abstract: The yeast (β4-S142F) mutant accumulates late 20S proteasome core particle precursor complexes (late-PCs). We report a 2.1 Å cryo-EM structure of this intermediate with full-length Ump1 trapped ...The yeast (β4-S142F) mutant accumulates late 20S proteasome core particle precursor complexes (late-PCs). We report a 2.1 Å cryo-EM structure of this intermediate with full-length Ump1 trapped inside, and Pba1-Pba2 attached to the α-ring surfaces. The structure discloses intimate interactions of Ump1 with β2- and β5-propeptides, which together fill most of the antechambers between the α- and β-rings. The β5-propeptide is unprocessed and separates Ump1 from β6 and β7. The β2-propeptide is disconnected from the subunit by autocatalytic processing and localizes between Ump1 and β3. A comparison of different proteasome maturation states reveals that maturation goes along with global conformational changes in the rings, initiated by structuring of the proteolytic sites and their autocatalytic activation. In the strain, β2 is activated first enabling processing of β1-, β6-, and β7-propeptides. Subsequent maturation of β5 and β1 precedes degradation of Ump1, tightening of the complex, and finally release of Pba1-Pba2. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_19523.map.gz emd_19523.map.gz | 777.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-19523-v30.xml emd-19523-v30.xml emd-19523.xml emd-19523.xml | 35.6 KB 35.6 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_19523_fsc.xml emd_19523_fsc.xml | 19.7 KB | Display |  FSC data file FSC data file |
| Images |  emd_19523.png emd_19523.png | 65.6 KB | ||
| Masks |  emd_19523_msk_1.map emd_19523_msk_1.map | 824 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-19523.cif.gz emd-19523.cif.gz | 9.5 KB | ||
| Others |  emd_19523_half_map_1.map.gz emd_19523_half_map_1.map.gz emd_19523_half_map_2.map.gz emd_19523_half_map_2.map.gz | 763.4 MB 763.4 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-19523 http://ftp.pdbj.org/pub/emdb/structures/EMD-19523 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19523 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19523 | HTTPS FTP |
-Validation report
| Summary document |  emd_19523_validation.pdf.gz emd_19523_validation.pdf.gz | 1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_19523_full_validation.pdf.gz emd_19523_full_validation.pdf.gz | 1 MB | Display | |
| Data in XML |  emd_19523_validation.xml.gz emd_19523_validation.xml.gz | 27.6 KB | Display | |
| Data in CIF |  emd_19523_validation.cif.gz emd_19523_validation.cif.gz | 35.6 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-19523 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-19523 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-19523 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-19523 | HTTPS FTP |
-Related structure data
| Related structure data |  8rvlMC  8rvoC  8rvpC  8rvqC  9gbkC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_19523.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_19523.map.gz / Format: CCP4 / Size: 824 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.834 Å | ||||||||||||||||||||||||||||||||||||
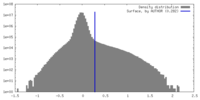
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_19523_msk_1.map emd_19523_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
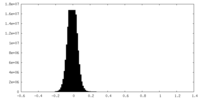
| Density Histograms |
-Half map: #2
| File | emd_19523_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
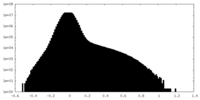
| Density Histograms |
-Half map: #1
| File | emd_19523_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Proteasomal late precursor complex from pre1-1
+Supramolecule #1: Proteasomal late precursor complex from pre1-1
+Macromolecule #1: Proteasome subunit beta type-7
+Macromolecule #2: Proteasome subunit alpha type-1
+Macromolecule #3: Proteasome subunit alpha type-4
+Macromolecule #4: Proteasome subunit alpha type-5
+Macromolecule #5: Proteasome subunit alpha type-6
+Macromolecule #6: Proteasome subunit beta type-1
+Macromolecule #7: Proteasome subunit beta type-6
+Macromolecule #8: Proteasome maturation factor UMP1
+Macromolecule #9: Proteasome chaperone 1
+Macromolecule #10: Proteasome assembly chaperone 2
+Macromolecule #11: Proteasome subunit alpha type-2
+Macromolecule #12: Proteasome subunit alpha type-3
+Macromolecule #13: Probable proteasome subunit alpha type-7
+Macromolecule #14: Proteasome subunit beta type-2
+Macromolecule #15: Proteasome subunit beta type-3
+Macromolecule #16: Proteasome subunit beta type-4
+Macromolecule #17: Proteasome subunit beta type-5
+Macromolecule #18: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.35 mg/mL |
|---|---|
| Buffer | pH: 7.5 / Details: 150 mM NaCl 50 mM Tris-HCl |
| Grid | Model: Quantifoil R2/4 / Material: COPPER / Mesh: 300 / Support film - Material: CARBON / Support film - topology: HOLEY / Pretreatment - Type: GLOW DISCHARGE / Pretreatment - Time: 30 sec. / Pretreatment - Atmosphere: OTHER / Pretreatment - Pressure: 0.02 kPa |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 80 % / Chamber temperature: 277 K / Instrument: HOMEMADE PLUNGER Details: manual plunge freezing device purchased from 'Neptune Fluid Flow Systems'. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Specialist optics | Phase plate: VOLTA PHASE PLATE |
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Number grids imaged: 1 / Number real images: 29904 / Average exposure time: 2.0 sec. / Average electron dose: 44.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 1.8 µm / Nominal defocus min: 0.6 µm / Nominal magnification: 105000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-8rvl: |
 Movie
Movie Controller
Controller






















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)