[English] 日本語
 Yorodumi
Yorodumi- EMDB-19388: Pseudo-symmetrical influenza B polymerase apo-dimer, ENDO(E) moie... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Pseudo-symmetrical influenza B polymerase apo-dimer, ENDO(E) moiety (from "Influenza B polymerase pseudo-symmetrical dimer" | Local refinement) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Viral polymerase / VIRAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationcap snatching / viral transcription / symbiont-mediated suppression of host mRNA transcription via inhibition of RNA polymerase II activity / 7-methylguanosine mRNA capping / host cell mitochondrion / virion component / endonuclease activity / Hydrolases; Acting on ester bonds / host cell cytoplasm / viral translational frameshifting ...cap snatching / viral transcription / symbiont-mediated suppression of host mRNA transcription via inhibition of RNA polymerase II activity / 7-methylguanosine mRNA capping / host cell mitochondrion / virion component / endonuclease activity / Hydrolases; Acting on ester bonds / host cell cytoplasm / viral translational frameshifting / symbiont-mediated suppression of host gene expression / RNA-directed RNA polymerase / viral RNA genome replication / RNA-directed RNA polymerase activity / nucleotide binding / DNA-templated transcription / host cell nucleus / RNA binding / metal ion binding Similarity search - Function | |||||||||
| Biological species |  Influenza B virus (B/Memphis/13/2003) Influenza B virus (B/Memphis/13/2003) | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 2.82 Å | |||||||||
 Authors Authors | Arragain B / Cusack S | |||||||||
| Funding support |  France, 1 items France, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Commun / Year: 2024 Journal: Nat Commun / Year: 2024Title: Structures of influenza A and B replication complexes give insight into avian to human host adaptation and reveal a role of ANP32 as an electrostatic chaperone for the apo-polymerase. Authors: Benoît Arragain / Tim Krischuns / Martin Pelosse / Petra Drncova / Martin Blackledge / Nadia Naffakh / Stephen Cusack /   Abstract: Replication of influenza viral RNA depends on at least two viral polymerases, a parental replicase and an encapsidase, and cellular factor ANP32. ANP32 comprises an LRR domain and a long C-terminal ...Replication of influenza viral RNA depends on at least two viral polymerases, a parental replicase and an encapsidase, and cellular factor ANP32. ANP32 comprises an LRR domain and a long C-terminal low complexity acidic region (LCAR). Here we present evidence suggesting that ANP32 is recruited to the replication complex as an electrostatic chaperone that stabilises the encapsidase moiety within apo-polymerase symmetric dimers that are distinct for influenza A and B polymerases. The ANP32 bound encapsidase, then forms the asymmetric replication complex with the replicase, which is embedded in a parental ribonucleoprotein particle (RNP). Cryo-EM structures reveal the architecture of the influenza A and B replication complexes and the likely trajectory of the nascent RNA product into the encapsidase. The cryo-EM map of the FluB replication complex shows extra density attributable to the ANP32 LCAR wrapping around and stabilising the apo-encapsidase conformation. These structures give new insight into the various mutations that adapt avian strain polymerases to use the distinct ANP32 in mammalian cells. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_19388.map.gz emd_19388.map.gz | 398.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-19388-v30.xml emd-19388-v30.xml emd-19388.xml emd-19388.xml | 20.4 KB 20.4 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_19388_fsc.xml emd_19388_fsc.xml | 15.8 KB | Display |  FSC data file FSC data file |
| Images |  emd_19388.png emd_19388.png | 51.4 KB | ||
| Masks |  emd_19388_msk_1.map emd_19388_msk_1.map | 421.9 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-19388.cif.gz emd-19388.cif.gz | 7.1 KB | ||
| Others |  emd_19388_additional_1.map.gz emd_19388_additional_1.map.gz emd_19388_half_map_1.map.gz emd_19388_half_map_1.map.gz emd_19388_half_map_2.map.gz emd_19388_half_map_2.map.gz | 211.4 MB 391.8 MB 391.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-19388 http://ftp.pdbj.org/pub/emdb/structures/EMD-19388 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19388 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-19388 | HTTPS FTP |
-Validation report
| Summary document |  emd_19388_validation.pdf.gz emd_19388_validation.pdf.gz | 1.1 MB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_19388_full_validation.pdf.gz emd_19388_full_validation.pdf.gz | 1.1 MB | Display | |
| Data in XML |  emd_19388_validation.xml.gz emd_19388_validation.xml.gz | 25 KB | Display | |
| Data in CIF |  emd_19388_validation.cif.gz emd_19388_validation.cif.gz | 32.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-19388 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-19388 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-19388 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-19388 | HTTPS FTP |
-Related structure data
| Related structure data |  8rn6MC  8rmpC  8rmqC  8rmrC  8rmsC  8rn0C  8rn1C  8rn2C  8rn3C  8rn4C  8rn5C  8rn7C  8rn8C  8rn9C  8rnaC  8rnbC  8rncC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_19388.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_19388.map.gz / Format: CCP4 / Size: 421.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.84 Å | ||||||||||||||||||||||||||||||||||||
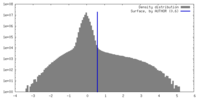
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_19388_msk_1.map emd_19388_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Additional map: #1
| File | emd_19388_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #1
| File | emd_19388_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: #2
| File | emd_19388_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Heterotrimeric complex from a pseudo-symmetrical dimer
| Entire | Name: Heterotrimeric complex from a pseudo-symmetrical dimer |
|---|---|
| Components |
|
-Supramolecule #1: Heterotrimeric complex from a pseudo-symmetrical dimer
| Supramolecule | Name: Heterotrimeric complex from a pseudo-symmetrical dimer type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:  Influenza B virus (B/Memphis/13/2003) Influenza B virus (B/Memphis/13/2003) |
-Macromolecule #1: Polymerase acidic protein
| Macromolecule | Name: Polymerase acidic protein / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: Hydrolases; Acting on ester bonds |
|---|---|
| Source (natural) | Organism:  Influenza B virus (B/Memphis/13/2003) Influenza B virus (B/Memphis/13/2003) |
| Molecular weight | Theoretical: 83.145023 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MDTFITRNFQ TTIIQKAKNT MAEFSEDPEL QPAMLFNICV HLEVCYVISD MNFLDEEGKA YTALEGQGKE QNLRPQYEVI EGMPRTIAW MVQRSLAQEH GIETPKYLAD LFDYKTKRFI EVGITKGLAD DYFWKKKEKL GNSMELMIFS YNQDYSLSNE S SLDEEGKG ...String: MDTFITRNFQ TTIIQKAKNT MAEFSEDPEL QPAMLFNICV HLEVCYVISD MNFLDEEGKA YTALEGQGKE QNLRPQYEVI EGMPRTIAW MVQRSLAQEH GIETPKYLAD LFDYKTKRFI EVGITKGLAD DYFWKKKEKL GNSMELMIFS YNQDYSLSNE S SLDEEGKG RVLSRLTELQ AELSLKNLWQ VLIGEEDVEK GIDFKLGQTI SRLRDISVPA GFSNFEGMRS YIDNIDPKGA IE RNLARMS PLVSVTPKKL TWEDLRPIGP HIYNHELPEV PYNAFLLMSD ELGLANMTEG KSKKPKTLAK ECLEKYSTLR DQT DPILIM KSEKANENFL WKLWRDCVNT ISNEEMSNEL QKTNYAKWAT GDGLTYQKIM KEVAIDDETM CQEEPKIPNK CRVA AWVQT EMNLLSTLTS KRALDLPEIG PDVAPVEHVG SERRKYFVNE INYCKASTVM MKYVLFHTSL LNESNASMGK YKVIP ITNR VVNEKGESFD MLYGLAVKGQ SHLRGDTDVV TVVTFEFSST DPRVDSGKWP KYTVFRIGSL FVSGREKSVY LYCRVN GTN KIQMKWGMEA RRCLLQSMQQ MEAIVEQESS IQGYDMTKAC FKGDRVNSPK TFSIGTQEGK LVKGSFGKAL RVIFTKC LM HYVFGNAQLE GFSAESRRLL LLIQALKDRK GPWVFDLEGM YSGIEECISN NPWVIQSAYW FNEWLGFEKE GSKVLESV D EIMDE UniProtKB: Polymerase acidic protein |
-Macromolecule #2: RNA-directed RNA polymerase catalytic subunit
| Macromolecule | Name: RNA-directed RNA polymerase catalytic subunit / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO / EC number: RNA-directed RNA polymerase |
|---|---|
| Source (natural) | Organism:  Influenza B virus (B/Memphis/13/2003) Influenza B virus (B/Memphis/13/2003) |
| Molecular weight | Theoretical: 84.433266 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MNINPYFLFI DVPIQAAIST TFPYTGVPPY SHGTGTGYTI DTVIRTHEYS NKGKQYISDV TGCTMVDPTN GPLPEDNEPS AYAQLDCVL EALDRMDEEH PGLFQAASQN AMETLMVTTV DKLTQGRQTF DWTVCRNQPA ATALNTTITS FRLNDLNGAD K GGLIPFCQ ...String: MNINPYFLFI DVPIQAAIST TFPYTGVPPY SHGTGTGYTI DTVIRTHEYS NKGKQYISDV TGCTMVDPTN GPLPEDNEPS AYAQLDCVL EALDRMDEEH PGLFQAASQN AMETLMVTTV DKLTQGRQTF DWTVCRNQPA ATALNTTITS FRLNDLNGAD K GGLIPFCQ DIIDSLDRPE MTFFSVKNIK KKLPAKNRKG FLIKRIPMKV KDKITKVEYI KRALSLNTMT KDAERGKLKR RA IATAGIQ IRGFVLVVEN LAKNICENLE QSGLPVGGNE KKAKLSNAVA KMLSNCPPGG ISMTVTGDNT KWNECLNPRI FLA MTERIT RDSPIWFRDF CSIAPVLFSN KIARLGKGFM ITSKTKRLKA QIPCPDLFSI PLERYNEETR AKLKKLKPFF NEEG TASLS PGMMMGMFNM LSTVLGVAAL GIKNIGNKEY LWDGLQSSDD FALFVNAKDE ETCMEGINDF YRTCKLLGIN MSKKK SYCN ETGMFEFTSM FYRDGFVSNF AMELPSFGVA GVNESADMAI GMTIIKNNMI NNGMGPATAQ TAIQLFIADY RYTYKC HRG DSKVEGKRMK IIKELWENTK GRDGLLVADG GPNIYNLRNL HIPEIVLKYN LMDPEYKGRL LHPQNPFVGH LSIEGIK EA DITPAHGPVK KMDYDAVSGT HSWRTKRNRS ILNTDQRNMI LEEQCYAKCC NLFEACFNSA SYRKPVGQHS MLEAMAHR L RMDARLDYES GRMSKDDFEK AMAHLGEIGY I UniProtKB: RNA-directed RNA polymerase catalytic subunit |
-Macromolecule #3: Polymerase basic protein 2
| Macromolecule | Name: Polymerase basic protein 2 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Influenza B virus (B/Memphis/13/2003) Influenza B virus (B/Memphis/13/2003) |
| Molecular weight | Theoretical: 90.95832 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MTLAKIELLK QLLRDNEAKT VLKQTTVDQY NIIRKFNTSR IEKNPSLRMK WAMCSNFPLA LTKGDMANRI PLEYKGIQLK TNAEDIGTK GQMCSIAAVT WWNTYGPIGD TEGFERVYES FFLRKMRLDN ATWGRITFGP VERVRKRVLL NPLTKEMPPD E ASNVIMEI ...String: MTLAKIELLK QLLRDNEAKT VLKQTTVDQY NIIRKFNTSR IEKNPSLRMK WAMCSNFPLA LTKGDMANRI PLEYKGIQLK TNAEDIGTK GQMCSIAAVT WWNTYGPIGD TEGFERVYES FFLRKMRLDN ATWGRITFGP VERVRKRVLL NPLTKEMPPD E ASNVIMEI LFPKEAGIPR ESTWIHRELI KEKREKLKGT MITPIVLAYM LERELVARRR FLPVAGATSA EFIEMLHCLQ GE NWRQIYH PGGNKLTESR SQSMIVACRK IIRRSIVASN PLELAVEIAN KTVIDTEPLK SCLAAIDGGD VACDIIRAAL GLK IRQRQR FGRLELKRIS GRGFKNDEEI LIGNGTIQKI GIWDGEEEFH VRCGECRGIL KKSKMKLEKL LINSAKKEDM RDLI ILCMV FSQDTRMFQG VRGEINFLNR AGQLLSPMYQ LQRYFLNRSN DLFDQWGYEE SPKASELHGI NESMNASDYT LKGVV VTRN VIDDFSSTET EKVSITKNLS LIKRTGEVIM GANDVSELES QAQLMITYDT PKMWEMGTTK ELVQNTYQWV LKNLVT LKA QFLLGKEDMF QWDAFEAFES IIPQKMAGQY SGFARAVLKQ MRDQEVMKTD QFIKLLPFCF SPPKLRSNGE PYQFLKL VL KGGGENFIEV RKGSPLFSYN PQTEVLTICG RMMSLKGKIE DEERNRSMGN AVLAGFLVSG KYDPDLGDFK TIEELEKL K PGEKANILLY QGKPVKVVKR KRYSALSNDI SQGIKRQRMT VESMGWALSG WSHPQFEKGG GSGGGSGGSA WSHPQFEK UniProtKB: Polymerase basic protein 2 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 8 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 40.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.0 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL |
|---|---|
| Output model |  PDB-8rn6: |
 Movie
Movie Controller
Controller



















 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)