+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Helical reconstruction of CHIKV nsP3 helical scaffolds | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Helical scaffold / Replication complex / Alpha granules / Viral factories / VIRAL PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationhost cell filopodium / ADP-ribose 1''-phosphate phosphatase / mRNA methyltransferase activity / mRNA 5'-triphosphate monophosphatase activity / mRNA 5'-phosphatase / polynucleotide 5'-phosphatase activity / poly(A) RNA polymerase activity / polynucleotide adenylyltransferase / regulation of cytoskeleton organization / symbiont-mediated suppression of host mRNA transcription via inhibition of RNA polymerase II activity ...host cell filopodium / ADP-ribose 1''-phosphate phosphatase / mRNA methyltransferase activity / mRNA 5'-triphosphate monophosphatase activity / mRNA 5'-phosphatase / polynucleotide 5'-phosphatase activity / poly(A) RNA polymerase activity / polynucleotide adenylyltransferase / regulation of cytoskeleton organization / symbiont-mediated suppression of host mRNA transcription via inhibition of RNA polymerase II activity / 7-methylguanosine mRNA capping / cysteine-type peptidase activity / symbiont-mediated suppression of host JAK-STAT cascade via inhibition of STAT1 activity / Transferases; Transferring one-carbon groups; Methyltransferases / host cell cytoplasmic vesicle membrane / Transferases; Transferring phosphorus-containing groups; Nucleotidyltransferases / nucleoside-triphosphate phosphatase / Hydrolases; Acting on peptide bonds (peptidases); Cysteine endopeptidases / RNA helicase activity / symbiont-mediated suppression of host type I interferon-mediated signaling pathway / RNA helicase / RNA-directed RNA polymerase / viral RNA genome replication / RNA-dependent RNA polymerase activity / virus-mediated perturbation of host defense response / DNA-templated transcription / host cell nucleus / GTP binding / host cell plasma membrane / ATP hydrolysis activity / proteolysis / RNA binding / ATP binding / membrane / metal ion binding Similarity search - Function | |||||||||
| Biological species |  Chikungunya virus strain S27-African prototype Chikungunya virus strain S27-African prototype | |||||||||
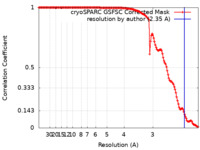
| Method | helical reconstruction / cryo EM / Resolution: 2.35 Å | |||||||||
 Authors Authors | Reguera J / Hons M / Zimberger C / Ptchelkine D / Jones R / Desfosses A | |||||||||
| Funding support |  France, 2 items France, 2 items
| |||||||||
 Citation Citation |  Journal: To be published Journal: To be publishedTitle: The alphavirus nsP3 protein forms helical tubular scaffolds important for viral replication and particle assembly Authors: Reguera J / Hons M / Zimberger C / Ptchelkine D / Jones R / Desfosses A | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_17678.map.gz emd_17678.map.gz | 244.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-17678-v30.xml emd-17678-v30.xml emd-17678.xml emd-17678.xml | 20.7 KB 20.7 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_17678_fsc.xml emd_17678_fsc.xml | 13.4 KB | Display |  FSC data file FSC data file |
| Images |  emd_17678.png emd_17678.png | 97.8 KB | ||
| Filedesc metadata |  emd-17678.cif.gz emd-17678.cif.gz | 6 KB | ||
| Others |  emd_17678_additional_1.map.gz emd_17678_additional_1.map.gz emd_17678_additional_2.map.gz emd_17678_additional_2.map.gz emd_17678_additional_3.map.gz emd_17678_additional_3.map.gz emd_17678_half_map_1.map.gz emd_17678_half_map_1.map.gz emd_17678_half_map_2.map.gz emd_17678_half_map_2.map.gz | 201.2 MB 128.3 MB 5.7 MB 239.8 MB 239.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-17678 http://ftp.pdbj.org/pub/emdb/structures/EMD-17678 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17678 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-17678 | HTTPS FTP |
-Related structure data
| Related structure data |  8phzMC  17729  8pk7C M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_17678.map.gz / Format: CCP4 / Size: 259.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_17678.map.gz / Format: CCP4 / Size: 259.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
- Sample components
Sample components
-Entire : Helical scaffold assembly of CHIKV nsP3 mediated by its Unique al...
| Entire | Name: Helical scaffold assembly of CHIKV nsP3 mediated by its Unique alphavirus domain. |
|---|---|
| Components |
|
-Supramolecule #1: Helical scaffold assembly of CHIKV nsP3 mediated by its Unique al...
| Supramolecule | Name: Helical scaffold assembly of CHIKV nsP3 mediated by its Unique alphavirus domain. type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Chikungunya virus strain S27-African prototype Chikungunya virus strain S27-African prototype |
| Molecular weight | Theoretical: 220.25 kDa/nm |
-Macromolecule #1: Non-structural protein 3
| Macromolecule | Name: Non-structural protein 3 / type: protein_or_peptide / ID: 1 / Number of copies: 5 / Enantiomer: LEVO / EC number: ADP-ribose 1''-phosphate phosphatase |
|---|---|
| Source (natural) | Organism:  Chikungunya virus strain S27-African prototype Chikungunya virus strain S27-African prototype |
| Molecular weight | Theoretical: 57.418207 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: APSYRVKRMD IAKNDEECVV NAANPRGLPG DGVCKAVYKK WPESFKNSAT PVGTAKTVMC GTYPVIHAVG PNFSNYSESE GDRELAAAY REVAKEVTRL GVNSVAIPLL STGVYSGGKD RLTQSLNHLF TAMDSTDADV VIYCRDKEWE KKISEAIQMR T QVELLDEH ...String: APSYRVKRMD IAKNDEECVV NAANPRGLPG DGVCKAVYKK WPESFKNSAT PVGTAKTVMC GTYPVIHAVG PNFSNYSESE GDRELAAAY REVAKEVTRL GVNSVAIPLL STGVYSGGKD RLTQSLNHLF TAMDSTDADV VIYCRDKEWE KKISEAIQMR T QVELLDEH ISIDCDVVRV HPDSSLAGRK GYSTTEGALY SYLEGTRFHQ TAVDMAEIYT MWPKQTEANE QVCLYALGES IE SIRQKCP VDDADASSPP KTVPCLCRYA MTPERVTRLR MNHVTSIIVC SSFPLPKYKI EGVQKVKCSK VMLFDHNVPS RVS PREYRP SQESVQEAST TTSLTHSQFD LSVDGKILPV PSDLDADAPA LEPALDDGAI HTLPSATGNL AAVSDWVMST VPVA PPRRR RGRNLTVTCD EREGNITPMA SVRFFRAELC PVVQETAETR DTAMSLQAPP STATELSHPP ISFGAPSETF PITFG DFNE GEIESLSSEL LTFGDFLPGE VDDLTDSDWS TCSDTDDEL UniProtKB: Polyprotein P1234 |
-Macromolecule #2: ZINC ION
| Macromolecule | Name: ZINC ION / type: ligand / ID: 2 / Number of copies: 5 / Formula: ZN |
|---|---|
| Molecular weight | Theoretical: 65.409 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | helical reconstruction |
| Aggregation state | helical array |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE-PROPANE |
| Details | his sample was heterogeneous in lenght |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Average electron dose: 18.1 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.5 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller