+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
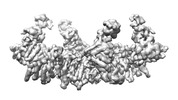
| Title | Cryo-EM map of ubiquitin-VME bound HECT E3 ligase UBR5 | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | E3 ligase / UBR5 / Ubiquitination / UBQ / Ubiquitin / HECT / K48 / UBQ-chain / polyubiquitylation / LIGASE | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
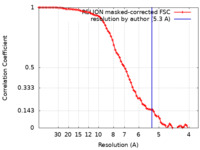
| Method | single particle reconstruction / cryo EM / Resolution: 5.3 Å | |||||||||
 Authors Authors | Hehl LA / Horn-Ghetko D / Prabu JR / Schulman BA | |||||||||
| Funding support |  Germany, 1 items Germany, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Chem Biol / Year: 2024 Journal: Nat Chem Biol / Year: 2024Title: Structural snapshots along K48-linked ubiquitin chain formation by the HECT E3 UBR5. Authors: Laura A Hehl / Daniel Horn-Ghetko / J Rajan Prabu / Ronnald Vollrath / D Tung Vu / David A Pérez Berrocal / Monique P C Mulder / Gerbrand J van der Heden van Noort / Brenda A Schulman /   Abstract: Ubiquitin (Ub) chain formation by homologous to E6AP C-terminus (HECT)-family E3 ligases regulates vast biology, yet the structural mechanisms remain unknown. We used chemistry and cryo-electron ...Ubiquitin (Ub) chain formation by homologous to E6AP C-terminus (HECT)-family E3 ligases regulates vast biology, yet the structural mechanisms remain unknown. We used chemistry and cryo-electron microscopy (cryo-EM) to visualize stable mimics of the intermediates along K48-linked Ub chain formation by the human E3, UBR5. The structural data reveal a ≈ 620 kDa UBR5 dimer as the functional unit, comprising a scaffold with flexibly tethered Ub-associated (UBA) domains, and elaborately arranged HECT domains. Chains are forged by a UBA domain capturing an acceptor Ub, with its K48 lured into the active site by numerous interactions between the acceptor Ub, manifold UBR5 elements and the donor Ub. The cryo-EM reconstructions allow defining conserved HECT domain conformations catalyzing Ub transfer from E2 to E3 and from E3. Our data show how a full-length E3, ubiquitins to be adjoined, E2 and intermediary products guide a feed-forward HECT domain conformational cycle establishing a highly efficient, broadly targeting, K48-linked Ub chain forging machine. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_16866.map.gz emd_16866.map.gz | 38.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-16866-v30.xml emd-16866-v30.xml emd-16866.xml emd-16866.xml | 13.3 KB 13.3 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_16866_fsc.xml emd_16866_fsc.xml | 8 KB | Display |  FSC data file FSC data file |
| Images |  emd_16866.png emd_16866.png | 62 KB | ||
| Masks |  emd_16866_msk_1.map emd_16866_msk_1.map | 42.9 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-16866.cif.gz emd-16866.cif.gz | 3.9 KB | ||
| Others |  emd_16866_half_map_1.map.gz emd_16866_half_map_1.map.gz emd_16866_half_map_2.map.gz emd_16866_half_map_2.map.gz | 33.1 MB 33.1 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-16866 http://ftp.pdbj.org/pub/emdb/structures/EMD-16866 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16866 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-16866 | HTTPS FTP |
-Validation report
| Summary document |  emd_16866_validation.pdf.gz emd_16866_validation.pdf.gz | 647.5 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_16866_full_validation.pdf.gz emd_16866_full_validation.pdf.gz | 647.1 KB | Display | |
| Data in XML |  emd_16866_validation.xml.gz emd_16866_validation.xml.gz | 13.9 KB | Display | |
| Data in CIF |  emd_16866_validation.cif.gz emd_16866_validation.cif.gz | 19.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16866 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16866 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16866 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-16866 | HTTPS FTP |
-Related structure data
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_16866.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_16866.map.gz / Format: CCP4 / Size: 42.9 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.885 Å | ||||||||||||||||||||||||||||||||||||
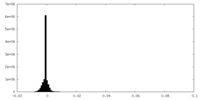
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_16866_msk_1.map emd_16866_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
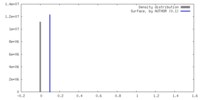
| Density Histograms |
-Half map: #2
| File | emd_16866_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
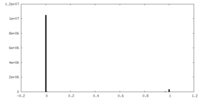
| Density Histograms |
-Half map: #1
| File | emd_16866_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
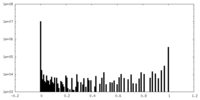
| Density Histograms |
- Sample components
Sample components
-Entire : Homo dimer of HECT E3 UBR5 cross-linked to Ub-VME
| Entire | Name: Homo dimer of HECT E3 UBR5 cross-linked to Ub-VME |
|---|---|
| Components |
|
-Supramolecule #1: Homo dimer of HECT E3 UBR5 cross-linked to Ub-VME
| Supramolecule | Name: Homo dimer of HECT E3 UBR5 cross-linked to Ub-VME / type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#4 / Details: Chemically synthesized Ub-VME |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 9 KDa |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS GLACIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Average electron dose: 60.0 e/Å2 |
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 2.6 µm / Nominal defocus min: 0.8 µm |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)