[English] 日本語
 Yorodumi
Yorodumi- EMDB-15473: Cryo-EM structure of crescentin filaments (wildtype, C2 symmetry ... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of crescentin filaments (wildtype, C2 symmetry and large box) | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | cytoskeleton / cell shape / intermediate filaments / coiled coil / assembly / STRUCTURAL PROTEIN | |||||||||
| Function / homology | :  Function and homology information Function and homology information | |||||||||
| Biological species |  Caulobacter vibrioides (bacteria) / Caulobacter vibrioides (bacteria) /  Camelidae (mammal) Camelidae (mammal) | |||||||||
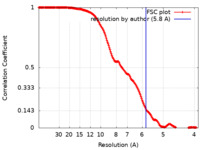
| Method | single particle reconstruction / cryo EM / Resolution: 5.8 Å | |||||||||
 Authors Authors | Liu Y / Lowe J | |||||||||
| Funding support |  United Kingdom, 1 items United Kingdom, 1 items
| |||||||||
 Citation Citation |  Journal: Proc Natl Acad Sci U S A / Year: 2024 Journal: Proc Natl Acad Sci U S A / Year: 2024Title: Filament structure and subcellular organization of the bacterial intermediate filament-like protein crescentin. Authors: Yue Liu / Fusinita van den Ent / Jan Löwe /  Abstract: The protein crescentin is required for the crescent shape of the freshwater bacterium (). Crescentin forms a filamentous structure on the inner, concave side of the curved cells. It shares features ...The protein crescentin is required for the crescent shape of the freshwater bacterium (). Crescentin forms a filamentous structure on the inner, concave side of the curved cells. It shares features with eukaryotic intermediate filament (IF) proteins, including the formation of static filaments based on long and parallel coiled coils, the protein's length, structural roles in cell and organelle shape determination and the presence of a coiled coil discontinuity called the "stutter." Here, we have used electron cryomicroscopy (cryo-EM) to determine the structure of the full-length protein and its filament, exploiting a crescentin-specific nanobody. The filament is formed by two strands, related by twofold symmetry, that each consist of two dimers, resulting in an octameric assembly. Crescentin subunits form longitudinal contacts head-to-head and tail-to-tail, making the entire filament non-polar. Using in vivo site-directed cysteine cross-linking, we demonstrated that contacts observed in the in vitro filament structure exist in cells. Electron cryotomography (cryo-ET) of cells expressing crescentin showed filaments on the concave side of the curved cells, close to the inner membrane, where they form a band. When comparing with current models of IF proteins and their filaments, which are also built from parallel coiled coil dimers and lack overall polarity, it emerges that IF proteins form head-to-tail longitudinal contacts in contrast to crescentin and hence several inter-dimer contacts in IFs have no equivalents in crescentin filaments. Our work supports the idea that intermediate filament-like proteins achieve their shared polymerization and mechanical properties through a variety of filament architectures. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_15473.map.gz emd_15473.map.gz | 422.6 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-15473-v30.xml emd-15473-v30.xml emd-15473.xml emd-15473.xml | 19 KB 19 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_15473_fsc.xml emd_15473_fsc.xml | 17.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_15473.png emd_15473.png | 38.9 KB | ||
| Masks |  emd_15473_msk_1.map emd_15473_msk_1.map | 476.8 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-15473.cif.gz emd-15473.cif.gz | 6.3 KB | ||
| Others |  emd_15473_half_map_1.map.gz emd_15473_half_map_1.map.gz emd_15473_half_map_2.map.gz emd_15473_half_map_2.map.gz | 443.2 MB 443.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-15473 http://ftp.pdbj.org/pub/emdb/structures/EMD-15473 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15473 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-15473 | HTTPS FTP |
-Validation report
| Summary document |  emd_15473_validation.pdf.gz emd_15473_validation.pdf.gz | 668.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_15473_full_validation.pdf.gz emd_15473_full_validation.pdf.gz | 668 KB | Display | |
| Data in XML |  emd_15473_validation.xml.gz emd_15473_validation.xml.gz | 24.3 KB | Display | |
| Data in CIF |  emd_15473_validation.cif.gz emd_15473_validation.cif.gz | 32.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15473 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15473 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15473 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-15473 | HTTPS FTP |
-Related structure data
| Related structure data |  8aixMC  8afeC  8afhC  8aflC  8afmC  8ahlC  8aiaC  8ajbC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_15473.map.gz / Format: CCP4 / Size: 476.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_15473.map.gz / Format: CCP4 / Size: 476.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.944 Å | ||||||||||||||||||||||||||||||||||||
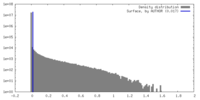
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Mask #1
| File |  emd_15473_msk_1.map emd_15473_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
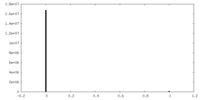
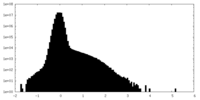
| Density Histograms |
-Half map: #1
| File | emd_15473_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
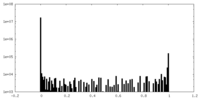
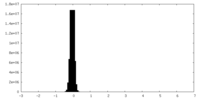
| Density Histograms |
-Half map: #2
| File | emd_15473_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
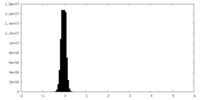
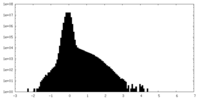
| Density Histograms |
- Sample components
Sample components
-Entire : Crescentin filaments in complex with megabodies
| Entire | Name: Crescentin filaments in complex with megabodies |
|---|---|
| Components |
|
-Supramolecule #1: Crescentin filaments in complex with megabodies
| Supramolecule | Name: Crescentin filaments in complex with megabodies / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|
-Supramolecule #2: Crescentin
| Supramolecule | Name: Crescentin / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Caulobacter vibrioides (bacteria) Caulobacter vibrioides (bacteria) |
-Supramolecule #3: Crescentin-specific megabody MB13
| Supramolecule | Name: Crescentin-specific megabody MB13 / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  Camelidae (mammal) Camelidae (mammal) |
-Macromolecule #1: Crescentin
| Macromolecule | Name: Crescentin / type: protein_or_peptide / ID: 1 / Number of copies: 16 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Caulobacter vibrioides (bacteria) Caulobacter vibrioides (bacteria) |
| Molecular weight | Theoretical: 49.918559 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MRLLSKNSRE TKNGKPTVLG DEARAEAMQH QIESTQAIGQ RYETIHGGLD SIGRVMEHLK AIEPLIAEIR GPVSQEFEAR RAEHAELIA VRANLDQAQR QIALIQAEER EVSARLAAAE TALGESDARR QTQDAALEDN ALEIDRLRNA LLQSDLKVSS L DASLRDAT ...String: MRLLSKNSRE TKNGKPTVLG DEARAEAMQH QIESTQAIGQ RYETIHGGLD SIGRVMEHLK AIEPLIAEIR GPVSQEFEAR RAEHAELIA VRANLDQAQR QIALIQAEER EVSARLAAAE TALGESDARR QTQDAALEDN ALEIDRLRNA LLQSDLKVSS L DASLRDAT ARIEHLVQDV EGLRVQAQDI DARRGDAEAA LARANQDNAL LGEEAATLKK RVDQAGLDLA RLSRIETDLE AQ LAAERAR VQAVENALAA HQADSGRTIR GLESQVEANR AEISALQTRL ETATGRADKL EEMNGQISAR LADSSAQQKA VER RAGDLN VALERALDRI RALEEEADGL RQRHAGVDTA RATAIERADQ LAKSAVAQEK ALKRAEERAQ QLRARLDAMQ EAQD QVRRD HEAKIAELQA TIERLTSEAA LAEGALEAAR RDRSRLQMAL LGASDGDVAA SA UniProtKB: UNIPROTKB: A0A8F8EC09 |
-Macromolecule #2: Crescentin-specific megabody MB13
| Macromolecule | Name: Crescentin-specific megabody MB13 / type: protein_or_peptide / ID: 2 / Number of copies: 8 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Camelidae (mammal) Camelidae (mammal) |
| Molecular weight | Theoretical: 101.79018 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: EVQLQESGGG LVYKEETQSG LNNYARVVEK GQYDSLEIPA QVAASWESGR DDAAVFGFID KEQLDKYVAN GGKRSDWTVK FAENRSQDG TLLGYSLLQE SVDQASYMYS DNHYLAEMAT ILGKPEEAKR YRQLAQQLAD YINTCMFDPT TQFYYDVRIE D KPLANGCA ...String: EVQLQESGGG LVYKEETQSG LNNYARVVEK GQYDSLEIPA QVAASWESGR DDAAVFGFID KEQLDKYVAN GGKRSDWTVK FAENRSQDG TLLGYSLLQE SVDQASYMYS DNHYLAEMAT ILGKPEEAKR YRQLAQQLAD YINTCMFDPT TQFYYDVRIE D KPLANGCA GKPIVERGKG PEGWSPLFNG AATQANADAV VKVMLDPKEF NTFVPLGTAA LTNPAFGADI YWRGRVWVDQ FW FGLKGME RYGYRDDALK LADTFFRHAK GLTADGPIQE NYNPLTGAQQ GAPNFSWSAA HLYMLYNDFF RKQASGGGSG GGG SGGGGS GNADNYKNVI NRTGAPQYMK DYDYDDHQRF NPFFDLGAWH GHLLPDGPNT MGGFPGVALL TEEYINFMAS NFDR LTVWQ DGKKVDFTLE AYSIPGALVQ KLTAKDVQVE MTLRFATPRT SLLETKITSN KPLDLVWDGE LLEKLEAKEG KPLSD KTIA GEYPDYQRKI SATRDGLKVT FGKVRATWDL LTSGESEYQV HKSLPVQTEI NGNRFTSKAH INGSTTLYTT YSHLLT AQE VSKEQMQIRD ILARPAFYLT ASQQRWEEYL KKGLTNPDAT PEQTRVAVKA IETLNGNWRS PGGAVKFNTV TPSVTGR WF SGNQTWPWDT WKQAFAMAHF NPDIAKENIR AVFSWQIQPG DSVRPQDVGF VPDLIAWNLS PERGGDGGNW NERNTKPS L AAWSVMEVYN VTQDKTWVAE MYPKLVAYHD WWLRNRDHNG NGVPEYGATR DKAHNTESGE MLFTVKKDSL RLSCASSRS IDGINIMRWY RQAPGKQRGM VAVVTGWGST NYVDSVKGRF IISRDSAKDT VYLQMNNLKP EDTAVYSCNA IYRGSEYWGQ GTQVTVSSG ENLYFQGSHH HHHHHHHH |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Concentration | 2 mg/mL |
|---|---|
| Buffer | pH: 6.5 / Details: PIPES 25mM, 0.05% CHAPS, pH 6.5 |
| Grid | Model: UltrAuFoil R2/2 / Material: GOLD / Mesh: 300 |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 283 K / Instrument: FEI VITROBOT MARK IV |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Temperature | Min: 80.0 K / Max: 80.0 K |
| Image recording | Film or detector model: FEI FALCON IV (4k x 4k) / Number grids imaged: 2 / Average exposure time: 10.0 sec. / Average electron dose: 34.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | C2 aperture diameter: 50.0 µm / Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Cs: 2.7 mm / Nominal defocus max: 3.5 µm / Nominal defocus min: 0.8 µm / Nominal magnification: 75000 |
| Sample stage | Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL |
|---|---|
| Output model |  PDB-8aix: |
 Movie
Movie Controller
Controller












 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)