[English] 日本語
 Yorodumi
Yorodumi- EMDB-14855: Mammalian Dicer in the "pre-dicing state" with pre-miR-15a substr... -
+ Open data
Open data
- Basic information
Basic information
| Entry | 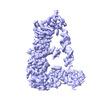 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Mammalian Dicer in the "pre-dicing state" with pre-miR-15a substrate and TARBP2 subunit | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | endoribonuclease / dsRNA / complex / gene silencing / post-transcriptional / catalytic complex / cytoplasm / RISC-loading complex / TARBP2 RNA BINDING PROTEIN / RNA BINDING PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationregulation of muscle cell apoptotic process / myoblast differentiation involved in skeletal muscle regeneration / MicroRNA (miRNA) biogenesis / Small interfering RNA (siRNA) biogenesis / regulation of siRNA processing / regulation of miRNA processing / ganglion development / hair follicle cell proliferation / zygote asymmetric cell division / regulation of oligodendrocyte differentiation ...regulation of muscle cell apoptotic process / myoblast differentiation involved in skeletal muscle regeneration / MicroRNA (miRNA) biogenesis / Small interfering RNA (siRNA) biogenesis / regulation of siRNA processing / regulation of miRNA processing / ganglion development / hair follicle cell proliferation / zygote asymmetric cell division / regulation of oligodendrocyte differentiation / cardiac neural crest cell development involved in outflow tract morphogenesis / positive regulation of endothelial cell-matrix adhesion via fibronectin / olfactory bulb interneuron differentiation / regulation of enamel mineralization / regulation of viral transcription / positive regulation of establishment of endothelial barrier / positive regulation of hepatic stellate cell proliferation / trophectodermal cell proliferation / regulation of miRNA metabolic process / regulation of odontogenesis of dentin-containing tooth / spermatogonial cell division / regulation of RNA metabolic process / regulation of regulatory ncRNA processing / peripheral nervous system myelin formation / negative regulation of defense response to virus by host / regulation of epithelial cell differentiation / pre-miRNA binding / regulation of regulatory T cell differentiation / regulation of Notch signaling pathway / spinal cord motor neuron differentiation / global gene silencing by mRNA cleavage / epidermis morphogenesis / negative regulation of Schwann cell proliferation / : / reproductive structure development / ribonuclease III / positive regulation of myelination / apoptotic DNA fragmentation / inner ear receptor cell development / skeletal muscle tissue regeneration / nerve development / meiotic spindle organization / positive regulation of Schwann cell differentiation / RISC-loading complex / deoxyribonuclease I activity / RISC complex assembly / intestinal epithelial cell development / ribonuclease III activity / regulatory ncRNA-mediated post-transcriptional gene silencing / pericentric heterochromatin formation / miRNA processing / regulation of stem cell differentiation / siRNA binding / pre-miRNA processing / regulation of viral genome replication / siRNA processing / neural precursor cell proliferation / digestive tract development / mRNA stabilization / embryonic hindlimb morphogenesis / cartilage development / cardiac muscle cell development / RISC complex / embryonic limb morphogenesis / miRNA binding / positive regulation of vascular endothelial cell proliferation / regulation of myelination / regulation of neuron differentiation / negative regulation of glial cell proliferation / hair follicle morphogenesis / stem cell population maintenance / branching morphogenesis of an epithelial tube / positive regulation of miRNA metabolic process / positive regulation of muscle cell differentiation / endoplasmic reticulum-Golgi intermediate compartment / spermatid development / regulation of neurogenesis / hair follicle development / single fertilization / positive regulation of collagen biosynthetic process / postsynaptic density, intracellular component / spindle assembly / positive regulation of viral genome replication / RNA processing / spleen development / positive regulation of endothelial cell migration / helicase activity / neuron projection morphogenesis / post-embryonic development / positive regulation of translation / regulation of protein phosphorylation / lung development / multicellular organism growth / cerebral cortex development / rRNA processing / double-stranded RNA binding / gene expression / growth cone / regulation of inflammatory response / regulation of gene expression Similarity search - Function | |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 3.81 Å | |||||||||
 Authors Authors | Zanova M / Zapletal D / Kubicek K / Stefl R / Pinkas M / Novacek J | |||||||||
| Funding support |  Czech Republic, 2 items Czech Republic, 2 items
| |||||||||
 Citation Citation |  Journal: Mol Cell / Year: 2022 Journal: Mol Cell / Year: 2022Title: Structural and functional basis of mammalian microRNA biogenesis by Dicer. Authors: David Zapletal / Eliska Taborska / Josef Pasulka / Radek Malik / Karel Kubicek / Martina Zanova / Christian Much / Marek Sebesta / Valeria Buccheri / Filip Horvat / Irena Jenickova / ...Authors: David Zapletal / Eliska Taborska / Josef Pasulka / Radek Malik / Karel Kubicek / Martina Zanova / Christian Much / Marek Sebesta / Valeria Buccheri / Filip Horvat / Irena Jenickova / Michaela Prochazkova / Jan Prochazka / Matyas Pinkas / Jiri Novacek / Diego F Joseph / Radislav Sedlacek / Carrie Bernecky / Dónal O'Carroll / Richard Stefl / Petr Svoboda /      Abstract: MicroRNA (miRNA) and RNA interference (RNAi) pathways rely on small RNAs produced by Dicer endonucleases. Mammalian Dicer primarily supports the essential gene-regulating miRNA pathway, but how it is ...MicroRNA (miRNA) and RNA interference (RNAi) pathways rely on small RNAs produced by Dicer endonucleases. Mammalian Dicer primarily supports the essential gene-regulating miRNA pathway, but how it is specifically adapted to miRNA biogenesis is unknown. We show that the adaptation entails a unique structural role of Dicer's DExD/H helicase domain. Although mice tolerate loss of its putative ATPase function, the complete absence of the domain is lethal because it assures high-fidelity miRNA biogenesis. Structures of murine Dicer•-miRNA precursor complexes revealed that the DExD/H domain has a helicase-unrelated structural function. It locks Dicer in a closed state, which facilitates miRNA precursor selection. Transition to a cleavage-competent open state is stimulated by Dicer-binding protein TARBP2. Absence of the DExD/H domain or its mutations unlocks the closed state, reduces substrate selectivity, and activates RNAi. Thus, the DExD/H domain structurally contributes to mammalian miRNA biogenesis and underlies mechanistical partitioning of miRNA and RNAi pathways. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
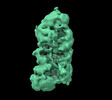
| Map data |  emd_14855.map.gz emd_14855.map.gz | 183.7 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-14855-v30.xml emd-14855-v30.xml emd-14855.xml emd-14855.xml | 24.6 KB 24.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_14855.png emd_14855.png | 79.7 KB | ||
| Filedesc metadata |  emd-14855.cif.gz emd-14855.cif.gz | 8.3 KB | ||
| Others |  emd_14855_half_map_1.map.gz emd_14855_half_map_1.map.gz emd_14855_half_map_2.map.gz emd_14855_half_map_2.map.gz | 199.8 MB 199.8 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-14855 http://ftp.pdbj.org/pub/emdb/structures/EMD-14855 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14855 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14855 | HTTPS FTP |
-Validation report
| Summary document |  emd_14855_validation.pdf.gz emd_14855_validation.pdf.gz | 610 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_14855_full_validation.pdf.gz emd_14855_full_validation.pdf.gz | 609.6 KB | Display | |
| Data in XML |  emd_14855_validation.xml.gz emd_14855_validation.xml.gz | 15.6 KB | Display | |
| Data in CIF |  emd_14855_validation.cif.gz emd_14855_validation.cif.gz | 18.5 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14855 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14855 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14855 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14855 | HTTPS FTP |
-Related structure data
| Related structure data |  7zpjMC  7yymC  7yynC  7yz4C  7zpiC  7zpkC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_14855.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_14855.map.gz / Format: CCP4 / Size: 216 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Voxel size | X=Y=Z: 0.835 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: #2
| File | emd_14855_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
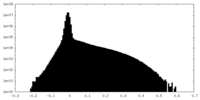
| Density Histograms |
-Half map: #1
| File | emd_14855_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Pre-dicing state of mouse somatic dicer with pre-mir-15a and TARBP2
| Entire | Name: Pre-dicing state of mouse somatic dicer with pre-mir-15a and TARBP2 |
|---|---|
| Components |
|
-Supramolecule #1: Pre-dicing state of mouse somatic dicer with pre-mir-15a and TARBP2
| Supramolecule | Name: Pre-dicing state of mouse somatic dicer with pre-mir-15a and TARBP2 type: complex / ID: 1 / Parent: 0 / Macromolecule list: #1-#3 |
|---|
-Supramolecule #2: Pre-dicing state of mouse somatic dicer with TARBP2
| Supramolecule | Name: Pre-dicing state of mouse somatic dicer with TARBP2 / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1, #3 |
|---|---|
| Source (natural) | Organism:  |
-Supramolecule #3: Pre-mir-15a
| Supramolecule | Name: Pre-mir-15a / type: complex / ID: 3 / Parent: 1 / Macromolecule list: #2 |
|---|---|
| Source (natural) | Organism:  |
-Macromolecule #1: Endoribonuclease Dicer
| Macromolecule | Name: Endoribonuclease Dicer / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO / EC number: ribonuclease III |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 226.925656 KDa |
| Recombinant expression | Organism:  Trichoplusia ni (cabbage looper) Trichoplusia ni (cabbage looper) |
| Sequence | String: MVWSHPQFEK GGGSGGGSGG SAWSHPQFEK GDYPYDVPDY AGTENLYFQG LVDMKSPALQ PLSMAGLQLM TPASSPMGPF FGLPWQQEA IHDNIYTPRK YQVELLEAAL DHNTIVCLNT GSGKTFIAVL LTKELAHQIR GDLNPHAKRT VFLVNSANQV A QQVSAVRT ...String: MVWSHPQFEK GGGSGGGSGG SAWSHPQFEK GDYPYDVPDY AGTENLYFQG LVDMKSPALQ PLSMAGLQLM TPASSPMGPF FGLPWQQEA IHDNIYTPRK YQVELLEAAL DHNTIVCLNT GSGKTFIAVL LTKELAHQIR GDLNPHAKRT VFLVNSANQV A QQVSAVRT HSDLKVGEYS DLEVNASWTK ERWSQEFTKH QVLIMTCYVA LTVLKNGYLS LSDINLLVFD ECHLAILDHP YR EIMKLCE SCPSCPRILG LTASILNGKC DPEELEEKIQ KLERILRSDA ETATDLVVLD RYTSQPCEIV VDCGPFTDRS GLY ERLLME LEAALDFIND CNVAVHSKER DSTLISKQIL SDCRAVLVVL GPWCADKVAG MMVRELQKYI KHEQEELHRK FLLF TDTLL RKIHALCEEY FSPASLDLKY VTPKVMKLLE ILRKYKPYER QQFESVEWYN NRNQDNYVSW SDSEDDDDDE EIEEK EKPE TNFPSPFTNI LCGIIFVERR YTAVVLNRLI KEAGKQDPEL AYISSNFITG HGIGKNQPRS KQMEAEFRKQ EEVLRK FRA HETNLLIATS VVEEGVDIPK CNLVVRFDLP TEYRSYVQSK GRARAPISNY VMLADTDKIK SFEEDLKTYK AIEKILR NK CSKSADGAEA DVHAGVDDED AFPPYVLRPD DGGPRVTINT AIGHINRYCA RLPSDPFTHL APKCRTRELP DGTFYSTL Y LPINSPLRAS IVGPPMDSVR LAERVVALIC CEKLHKIGEL DEHLMPVGKE TVKYEEELDL HDEEETSVPG RPGSTKRRQ CYPKAIPECL RESYPKPDQP CYLYVIGMVL TTPLPDELNF RRRKLYPPED TTRCFGILTA KPIPQIPHFP VYTRSGEVTI SIELKKSGF TLSQQMLELI TRLHQYIFSH ILRLEKPALE FKPTGAESAY CVLPLNVVND SGTLDIDFKF MEDIEKSEAR I GIPSTKYS KETPFVFKLE DYQDAVIIPR YRNFDQPHRF YVADVYTDLT PLSKFPSPEY ETFAEYYKTK YNLDLTNLNQ PL LDVDHTS SRLNLLTPRH LNQKGKALPL SSAEKRKAKW ESLQNKQILV PELCAIHPIP ASLWRKAVCL PSILYRLHCL LTA EELRAQ TASDAGVGVR SLPVDFRYPN LDFGWKKSID SKSFISSCNS SLAESDNYCK HSTTVVPEHA AHQGATRPSL ENHD QMSVN CKRLPAESPA KLQSEVSTDL TAINGLSYNK NLANGSYDLV NRDFCQGNQL NYFKQEIPVQ PTTSYPIQNL YNYEN QPKP SNECPLLSNT YLDGNANTST SDGSPAVSTM PAMMNAVKAL KDRMDSEQSP SVGYSSRTLG PNPGLILQAL TLSNAS DGF NLERLEMLGD SFLKHAITTY LFCTYPDAHE GRLSYMRSKK VSNCNLYRLG KKKGLPSRMV VSIFDPPVNW LPPGYVV NQ DKSNSEKWEK DEMTKDCLLA NGKLGEACEE EEDLTWRAPK EEAEDEDDFL EYDQEHIQFI DSMLMGSGAF VRKISLSP F SASDSAYEWK MPKKASLGSM PFASGLEDFD YSSWDAMCYL DPSKAVEEDD FVVGFWNPSE ENCGVDTGKQ SISYDLHTE QCIADKSIAD CVEALLGCYL TSCGERAAQL FLCSLGLKVL PVIKRTSREK ALDPAQENGS SQQKSLSGSC ASPVGPRSSA GKDLEYGCL KIPPRCMFDH PDAEKTLNHL ISGFETFEKK INYRFKNKAY LLQAFTHASY HYNTITDCYQ RLEFLGDAIL D YLITKHLY EDPRQHSPGV LTDLRSALVN NTIFASLAVK YDYHKYFKAV SPELFHVIDD FVKFQLEKNE MQGMDSELRR SE EDEEKEE DIEVPKAMGD IFESLAGAIY MDSGMSLEVV WQVYYPMMQP LIEKFSANVP RSPVRELLEM EPETAKFSPA ERT YDGKVR VTVEVVGKGK FKGVGRSYRI AKSAAARRAL RSLKANQPQV PNSGRGENLY FQGASDYKDH DGDYKDHDGS HHHH HHHH UniProtKB: Endoribonuclease Dicer |
-Macromolecule #3: RISC-loading complex subunit TARBP2 isoform 1
| Macromolecule | Name: RISC-loading complex subunit TARBP2 isoform 1 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 39.991059 KDa |
| Recombinant expression | Organism:  |
| Sequence | String: MSEEDQGSGT TTGCGLPSIE QMLAANPGKT PISLLQEYGT RIGKTPVYDL LKAEGQAHQP NFTFRVTVGD TSCTGQGPSK KAAKHKAAE VALKHLKGGS MLEPALEDSS SFSLLDSSPP EDTPVVAAEA AAPVPSAVLT RSPPMEMQPP VSPQQSECNP V GALQELVV ...String: MSEEDQGSGT TTGCGLPSIE QMLAANPGKT PISLLQEYGT RIGKTPVYDL LKAEGQAHQP NFTFRVTVGD TSCTGQGPSK KAAKHKAAE VALKHLKGGS MLEPALEDSS SFSLLDSSPP EDTPVVAAEA AAPVPSAVLT RSPPMEMQPP VSPQQSECNP V GALQELVV QKGWRLPEYM VTQESGPAHR KEFTMTCRVE RFIEIGSGTS KKLAKRNAAA KMLLRVHTVP LDARDGNEAE PD DDHFSIG VSSRLDGLRN RGPGCTWDSL RNSVGEKILS LRSCSVGSLG ALGSACCSVL SELSEEQAFH VSYLDIEELS LSG LCQCLV ELSTQPATVC YGSATTREAA RGDAAHRALQ YLRIMAGSKH HHHHHHH UniProtKB: RISC-loading complex subunit TARBP2 |
-Macromolecule #2: 59-nt precursor of miR-15a
| Macromolecule | Name: 59-nt precursor of miR-15a / type: rna / ID: 2 / Number of copies: 1 |
|---|---|
| Source (natural) | Organism:  |
| Molecular weight | Theoretical: 19.017279 KDa |
| Sequence | String: UAGCAGCACA UAAUGGUUUG UGGAUGUUGA AAAGGUGCAG GCCAUACUGU GCUGCCUCA GENBANK: GENBANK: NR_029733.1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.2 mg/mL | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 8 Component:
Details: The buffer was always prepared fresh in RNAse-free manner. | ||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 277.15 K / Instrument: FEI VITROBOT MARK IV / Details: Described in STAR methods. |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) / Detector mode: COUNTING / Number real images: 48253 / Average electron dose: 60.198 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 3.5 µm / Nominal defocus min: 0.8 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||
|---|---|---|---|---|---|---|---|
| Details | We used our previous deposition 7YYM as an initial model source. TARBP2 initial coordinates were predicted by AlphaFold. Initial local fitting was done using Chimera and then Coot's Real Space Refine Zone. PHENIX Real-space refinement was used for flexible fitting. ISOLDE was used for flexible fitting with torsion restraints defined for polypeptide chain and distance restraints for polyribonucleotides. | ||||||
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Overall B value: 189.2 Target criteria: Ramachandran Plot, Rotamer Analysis, Density Fit Analysis, Correlation coefficient | ||||||
| Output model |  PDB-7zpj: |
 Movie
Movie Controller
Controller









 Z
Z Y
Y X
X