[English] 日本語
 Yorodumi
Yorodumi- EMDB-14089: Portal protein assembly of the phicrAss001 virion with C12 symmet... -
+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Portal protein assembly of the phicrAss001 virion with C12 symmetry imposed | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | crAssphage / bacteriophage / virus / DNA virus / portal / connector | |||||||||
| Function / homology |  Function and homology information Function and homology informationvirus tail / host cell membrane / virion component / viral capsid / symbiont entry into host cell / membrane Similarity search - Function | |||||||||
| Biological species |  Bacteroides phage crAss001 (virus) Bacteroides phage crAss001 (virus) | |||||||||
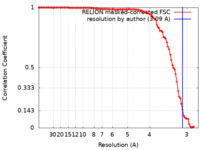
| Method | single particle reconstruction / cryo EM / Resolution: 3.09 Å | |||||||||
 Authors Authors | Bayfield OW / Shkoporov AN / Yutin N / Khokhlova EV / Smith JLR / Hawkins DEDP / Koonin EV / Hill C / Antson AA | |||||||||
| Funding support |  United Kingdom, 1 items United Kingdom, 1 items
| |||||||||
 Citation Citation |  Journal: Nature / Year: 2023 Journal: Nature / Year: 2023Title: Structural atlas of a human gut crassvirus. Authors: Oliver W Bayfield / Andrey N Shkoporov / Natalya Yutin / Ekaterina V Khokhlova / Jake L R Smith / Dorothy E D P Hawkins / Eugene V Koonin / Colin Hill / Alfred A Antson /    Abstract: CrAssphage and related viruses of the order Crassvirales (hereafter referred to as crassviruses) were originally discovered by cross-assembly of metagenomic sequences. They are the most abundant ...CrAssphage and related viruses of the order Crassvirales (hereafter referred to as crassviruses) were originally discovered by cross-assembly of metagenomic sequences. They are the most abundant viruses in the human gut, are found in the majority of individual gut viromes, and account for up to 95% of the viral sequences in some individuals. Crassviruses are likely to have major roles in shaping the composition and functionality of the human microbiome, but the structures and roles of most of the virally encoded proteins are unknown, with only generic predictions resulting from bioinformatic analyses. Here we present a cryo-electron microscopy reconstruction of Bacteroides intestinalis virus ΦcrAss001, providing the structural basis for the functional assignment of most of its virion proteins. The muzzle protein forms an assembly about 1 MDa in size at the end of the tail and exhibits a previously unknown fold that we designate the 'crass fold', that is likely to serve as a gatekeeper that controls the ejection of cargos. In addition to packing the approximately 103 kb of virus DNA, the ΦcrAss001 virion has extensive storage space for virally encoded cargo proteins in the capsid and, unusually, within the tail. One of the cargo proteins is present in both the capsid and the tail, suggesting a general mechanism for protein ejection, which involves partial unfolding of proteins during their extrusion through the tail. These findings provide a structural basis for understanding the mechanisms of assembly and infection of these highly abundant crassviruses. #1:  Journal: Res Sq / Year: 2023 Journal: Res Sq / Year: 2023Title: Structural atlas of the most abundant human gut virus Authors: Antson A / Bayfield O / Shkoporov A / Yutin N / Khokhlova E / Smith J / Hawkins D / Koonin E / Hill C | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_14089.map.gz emd_14089.map.gz | 52.2 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-14089-v30.xml emd-14089-v30.xml emd-14089.xml emd-14089.xml | 19.8 KB 19.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_14089_fsc.xml emd_14089_fsc.xml | 10.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_14089.png emd_14089.png | 107.9 KB | ||
| Filedesc metadata |  emd-14089.cif.gz emd-14089.cif.gz | 6.6 KB | ||
| Others |  emd_14089_additional_1.map.gz emd_14089_additional_1.map.gz emd_14089_additional_2.map.gz emd_14089_additional_2.map.gz | 11.2 MB 82.2 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-14089 http://ftp.pdbj.org/pub/emdb/structures/EMD-14089 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14089 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-14089 | HTTPS FTP |
-Validation report
| Summary document |  emd_14089_validation.pdf.gz emd_14089_validation.pdf.gz | 573 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_14089_full_validation.pdf.gz emd_14089_full_validation.pdf.gz | 572.6 KB | Display | |
| Data in XML |  emd_14089_validation.xml.gz emd_14089_validation.xml.gz | 11.2 KB | Display | |
| Data in CIF |  emd_14089_validation.cif.gz emd_14089_validation.cif.gz | 14.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14089 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14089 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14089 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-14089 | HTTPS FTP |
-Related structure data
| Related structure data |  7qogMC  7qofC  7qohC  7qoiC  7qojC  7qokC  7qolC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_14089.map.gz / Format: CCP4 / Size: 89.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_14089.map.gz / Format: CCP4 / Size: 89.2 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.3941 Å | ||||||||||||||||||||||||||||||||||||
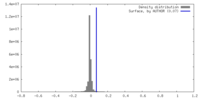
| Density |
| ||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Additional map: #1
| File | emd_14089_additional_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
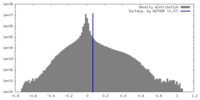
| Density Histograms |
-Additional map: #2
| File | emd_14089_additional_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
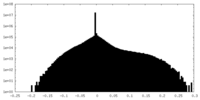
| Density Histograms |
- Sample components
Sample components
-Entire : Bacteroides phage crAss001
| Entire | Name:  Bacteroides phage crAss001 (virus) Bacteroides phage crAss001 (virus) |
|---|---|
| Components |
|
-Supramolecule #1: Bacteroides phage crAss001
| Supramolecule | Name: Bacteroides phage crAss001 / type: virus / ID: 1 / Parent: 0 / Macromolecule list: all / NCBI-ID: 2301731 / Sci species name: Bacteroides phage crAss001 / Virus type: VIRION / Virus isolate: SPECIES / Virus enveloped: No / Virus empty: No |
|---|
-Macromolecule #1: Portal protein gp20
| Macromolecule | Name: Portal protein gp20 / type: protein_or_peptide / ID: 1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Bacteroides phage crAss001 (virus) Bacteroides phage crAss001 (virus) |
| Molecular weight | Theoretical: 93.122211 KDa |
| Sequence | String: MADFLNFPRQ MLPFSKKTKQ WRKDCLLWAN QKTFFNYSLV RKSVIHKKIN YDLLNGRLHM SDLELVLNPD GIKAAYIPDR LQHYPIMNS KLNVLRGEES KRVFDFKVVV TNPNAISEIE DNKKNELLQR LQEMITDTSI SEDEYNIKLE KLNDYYTYEW Q DIREVRAN ...String: MADFLNFPRQ MLPFSKKTKQ WRKDCLLWAN QKTFFNYSLV RKSVIHKKIN YDLLNGRLHM SDLELVLNPD GIKAAYIPDR LQHYPIMNS KLNVLRGEES KRVFDFKVVV TNPNAISEIE DNKKNELLQR LQEMITDTSI SEDEYNIKLE KLNDYYTYEW Q DIREVRAN ELLNHYIKEY DIPLIFNNGF MDAMTCGEEI YQCDIVGGEP VIERVNPLKI RIFKSGYSNK VEDADMIILE DY WSPGRVI DTYYDVLSPK DIKYIETMPD YIGQGAVDQM DNIDERYGFV NQNMIGDEIT VRDGTYFFDP ANLFTEGIAN SLL PYDLAG NLRVLRLYWK SKRKILKVKS YDPETGEEEW NFYPENYVVN KEAGEEVQSF WVNEAWEGTM IGNEIFVNMR PRLI QYNRL NNPSRCHFGI VGSIYNLNDS RPFSLVDMMK PYNYLYDAIH DRLNKAIASN WGSILELDLS KVPKGWDVGK WMYYA RVNH IAVIDSFKEG TIGASTGKLA GALNNAGKGM IETNIGNYIQ QQINLLEFIK MEMADVAGIS KQREGQISQR ETVGGV ERA TLQSSHITEW LFTIHDDVKK RALECFLETA KVALKGRNKK FQYILSDTST RVMEIDGDEF AEADYGLVVD NSNGTQE LQ QKLDTLAQAA LQTQTLSFST ITKLYTSSSL AEKQRLIEKD EKQIRERQAQ AQKEQLEAQQ QIAAMQQQQK EAELLQKE E ANIRDNQTKI IIAQIQSEGG PDEEDGIMID DYSPEAKANL AEKIREFDEK LKLDKDKLKL DKKKAETDAS IKRQALRKK SSTTNK UniProtKB: Portal protein |
-Macromolecule #2: Ring protein 1 gp43
| Macromolecule | Name: Ring protein 1 gp43 / type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Bacteroides phage crAss001 (virus) Bacteroides phage crAss001 (virus) |
| Molecular weight | Theoretical: 27.479625 KDa |
| Sequence | String: MVNNINWVKL PVILDRLLRH PLLTDLNLET AIQYTLDFIS AMGLPNVYVD KIETIDIKEY RGELPCDLIS INQVRLHKNG IALRAMTDN FNAYPTHDHK EGDWYERGEP SFKTQGRVIF TSIKHEKVDI SYKAIMLDDE GLPLIPDNPI FLKTLELYIK K EWFTILFD ...String: MVNNINWVKL PVILDRLLRH PLLTDLNLET AIQYTLDFIS AMGLPNVYVD KIETIDIKEY RGELPCDLIS INQVRLHKNG IALRAMTDN FNAYPTHDHK EGDWYERGEP SFKTQGRVIF TSIKHEKVDI SYKAIMLDDE GLPLIPDNPI FLKTLELYIK K EWFTILFD MGKISPAVLN NTQQEYAFKA GQCNNEFVIP SVSEMEAITN MWNQLIPRVT EFRRGFKNLG DKEYIRVH UniProtKB: Ring protein 1 |
-Macromolecule #3: Ring protein 2 gp40
| Macromolecule | Name: Ring protein 2 gp40 / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Bacteroides phage crAss001 (virus) Bacteroides phage crAss001 (virus) |
| Molecular weight | Theoretical: 26.216832 KDa |
| Sequence | String: MTYNELIYMV LDELKLSSDD SYYTPDHVIF LLVKYRSFLL KQRYSDIKKQ IPDSDYQSIC LDLIEVPAIS GEPCEGSSYL RSKNKVPTT MMIGNPRVYP MDFYQGEITY ISRDRMRYVG YNKFLRNIIY CSKAPDGYLY FKSWNPQFLH LEKVSFNAIF E DAKEASEM ...String: MTYNELIYMV LDELKLSSDD SYYTPDHVIF LLVKYRSFLL KQRYSDIKKQ IPDSDYQSIC LDLIEVPAIS GEPCEGSSYL RSKNKVPTT MMIGNPRVYP MDFYQGEITY ISRDRMRYVG YNKFLRNIIY CSKAPDGYLY FKSWNPQFLH LEKVSFNAIF E DAKEASEM ACPEENGTIC KLEDKEFPIE DALVPPLIEL VVKELRGPEY SPKDEDNNAK DDLPDAR UniProtKB: Ring protein 2 |
-Macromolecule #4: Cargo protein 1 gp45
| Macromolecule | Name: Cargo protein 1 gp45 / type: protein_or_peptide / ID: 4 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Bacteroides phage crAss001 (virus) Bacteroides phage crAss001 (virus) |
| Molecular weight | Theoretical: 90.913836 KDa |
| Sequence | String: MAKKKIKRRG KMPPNIFDTG GQSWGQQSSG QFSNAFKGEN LGNSIGSIGG AVGGIAQAGI SNAQIADTSG IEAQNKAQKN MVVGASSND DLMSEWGSWN KVKDDYSWKD VRGGSTGQRV TNTIGAAGQG AAAGASVGGP IGAIVGGVVG LGSAIGGWLG G NRKAKRKA ...String: MAKKKIKRRG KMPPNIFDTG GQSWGQQSSG QFSNAFKGEN LGNSIGSIGG AVGGIAQAGI SNAQIADTSG IEAQNKAQKN MVVGASSND DLMSEWGSWN KVKDDYSWKD VRGGSTGQRV TNTIGAAGQG AAAGASVGGP IGAIVGGVVG LGSAIGGWLG G NRKAKRKA KKLNKEAKEA NERALTSFET RADNIDTQND FNMLANFSAY GGPLEFGSGA IGYEFDNRYL NNQEMSAVAK QR LTSLPNS FQALPEMNTY NAFAEGGGLS REKNYGSKKK PYPSVPSGDF AGPHRSYPIP TKADARDALR LAGLHGNESV RRK VLAKYP SLKAFGGSLF DSVVGNNFNQ SFTQGIQGMF QQEPEQTVQA ANIAKDGGDI KIKEKNKGKF TAYCGGKVTE ACIR KGKNS SNPTTRKRAT FAQNARNWNA FGGWLNTQGG DFTNGVTFIN EGGSHEENPY QGIQIGVDPE GAPNLVEQGE VVYDD YVFS DRMEIPDDIR KEYKLRGKTF AKAAKSAQRE SEERPNDPLS TKGLQAAMER IATAQEEARQ RKEAHREGNE YPSMFA YGG DTNPYGLALE DPMSVEELEA LMVQSGETGE IAPEGNNGNR QTWTRYAPII GSGLASLSDL FSKPDYDSAD LISGVDL GA EAVGYAPIGN YLSYRPLDRD FYINKMNQQA AATRRGLMNT SGGNRLNAQA GILAADYNYG QNMGNLARQA EEYNQQLR E RVEAFNRGTN MFNTETGLKA SMFNAESRNA AKRARLGQAT TVAQLRQGIK DQDAARRSAN ITNFLQGLGD MGWENEQAN WLDTLAKSGV LKMNTKGEYT GGTKKAKGGK VRTKKKKGLT YG UniProtKB: Cargo protein 1 |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Image recording | Film or detector model: FEI FALCON III (4k x 4k) / Detector mode: INTEGRATING / Average electron dose: 51.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal defocus max: 1.5 µm / Nominal defocus min: 0.3 µm |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
+ Image processing
Image processing
-Atomic model buiding 1
| Refinement | Space: REAL / Protocol: AB INITIO MODEL / Overall B value: 172 |
|---|---|
| Output model |  PDB-7qog: |
 Movie
Movie Controller
Controller










 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)