[English] 日本語
 Yorodumi
Yorodumi- EMDB-12607: Human TRiC complex in closed state with nanobody and tubulin bound -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12607 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Human TRiC complex in closed state with nanobody and tubulin bound | |||||||||
 Map data Map data | ||||||||||
 Sample Sample |
| |||||||||
| Function / homology |  Function and homology information Function and homology informationPost-chaperonin tubulin folding pathway / zona pellucida receptor complex / Microtubule-dependent trafficking of connexons from Golgi to the plasma membrane / Cilium Assembly / scaRNA localization to Cajal body / Carboxyterminal post-translational modifications of tubulin / chaperone mediated protein folding independent of cofactor / positive regulation of establishment of protein localization to telomere / positive regulation of protein localization to Cajal body / tubulin complex assembly ...Post-chaperonin tubulin folding pathway / zona pellucida receptor complex / Microtubule-dependent trafficking of connexons from Golgi to the plasma membrane / Cilium Assembly / scaRNA localization to Cajal body / Carboxyterminal post-translational modifications of tubulin / chaperone mediated protein folding independent of cofactor / positive regulation of establishment of protein localization to telomere / positive regulation of protein localization to Cajal body / tubulin complex assembly / BBSome-mediated cargo-targeting to cilium / Sealing of the nuclear envelope (NE) by ESCRT-III / chaperonin-containing T-complex / Intraflagellar transport / binding of sperm to zona pellucida / positive regulation of telomerase RNA localization to Cajal body / Folding of actin by CCT/TriC / Formation of tubulin folding intermediates by CCT/TriC / COPI-independent Golgi-to-ER retrograde traffic / Gap junction assembly / Prefoldin mediated transfer of substrate to CCT/TriC / Kinesins / Assembly and cell surface presentation of NMDA receptors / RHOBTB1 GTPase cycle / intermediate filament cytoskeleton / COPI-dependent Golgi-to-ER retrograde traffic / WD40-repeat domain binding / intercellular bridge / pericentriolar material / beta-tubulin binding / : / Association of TriC/CCT with target proteins during biosynthesis / Recycling pathway of L1 / chaperone-mediated protein complex assembly / RHO GTPases activate IQGAPs / RHOBTB2 GTPase cycle / Hedgehog 'off' state / COPI-mediated anterograde transport / heterochromatin / Activation of AMPK downstream of NMDARs / Mitotic Prometaphase / chaperone-mediated protein folding / EML4 and NUDC in mitotic spindle formation / Recruitment of NuMA to mitotic centrosomes / protein folding chaperone / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / positive regulation of telomere maintenance via telomerase / MHC class II antigen presentation / Resolution of Sister Chromatid Cohesion / Gene and protein expression by JAK-STAT signaling after Interleukin-12 stimulation / acrosomal vesicle / cell projection / mRNA 3'-UTR binding / Translocation of SLC2A4 (GLUT4) to the plasma membrane / RHO GTPases Activate Formins / ATP-dependent protein folding chaperone / response to virus / PKR-mediated signaling / cilium / structural constituent of cytoskeleton / cerebral cortex development / mitotic spindle / mRNA 5'-UTR binding / microtubule cytoskeleton organization / Aggrephagy / HCMV Early Events / Separation of Sister Chromatids / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / The role of GTSE1 in G2/M progression after G2 checkpoint / azurophil granule lumen / microtubule cytoskeleton / G-protein beta-subunit binding / unfolded protein binding / extracellular vesicle / melanosome / protein folding / mitotic cell cycle / cell body / secretory granule lumen / ficolin-1-rich granule lumen / microtubule / cytoskeleton / protein stabilization / cadherin binding / GTPase activity / centrosome / ubiquitin protein ligase binding / Neutrophil degranulation / GTP binding / Golgi apparatus / ATP hydrolysis activity / RNA binding / extracellular exosome / extracellular region / nucleoplasm / ATP binding / nucleus / metal ion binding / cytosol / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) / Homo sapiens (human) /   Human (human) Human (human) | |||||||||
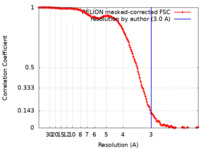
| Method | single particle reconstruction / cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Kelly JJ / Chi G / Bulawa C / Paavilainen VO / Bountra C / Huiskonen JT / Yue W | |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2022 Journal: Nat Struct Mol Biol / Year: 2022Title: Snapshots of actin and tubulin folding inside the TRiC chaperonin. Authors: John J Kelly / Dale Tranter / Els Pardon / Gamma Chi / Holger Kramer / Lotta Happonen / Kelly M Knee / Jay M Janz / Jan Steyaert / Christine Bulawa / Ville O Paavilainen / Juha T Huiskonen / Wyatt W Yue /      Abstract: The integrity of a cell's proteome depends on correct folding of polypeptides by chaperonins. The chaperonin TCP-1 ring complex (TRiC) acts as obligate folder for >10% of cytosolic proteins, ...The integrity of a cell's proteome depends on correct folding of polypeptides by chaperonins. The chaperonin TCP-1 ring complex (TRiC) acts as obligate folder for >10% of cytosolic proteins, including he cytoskeletal proteins actin and tubulin. Although its architecture and how it recognizes folding substrates are emerging from structural studies, the subsequent fate of substrates inside the TRiC chamber is not defined. We trapped endogenous human TRiC with substrates (actin, tubulin) and cochaperone (PhLP2A) at different folding stages, for structure determination by cryo-EM. The already-folded regions of client proteins are anchored at the chamber wall, positioning unstructured regions toward the central space to achieve their native fold. Substrates engage with different sections of the chamber during the folding cycle, coupled to TRiC open-and-close transitions. Further, the cochaperone PhLP2A modulates folding, acting as a molecular strut between substrate and TRiC chamber. Our structural snapshots piece together an emerging model of client protein folding within TRiC. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12607.map.gz emd_12607.map.gz | 228.5 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12607-v30.xml emd-12607-v30.xml emd-12607.xml emd-12607.xml | 29.8 KB 29.8 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_12607_fsc.xml emd_12607_fsc.xml | 14.2 KB | Display |  FSC data file FSC data file |
| Images |  emd_12607.png emd_12607.png | 215.6 KB | ||
| Masks |  emd_12607_msk_1.map emd_12607_msk_1.map | 244.1 MB |  Mask map Mask map | |
| Others |  emd_12607_half_map_1.map.gz emd_12607_half_map_1.map.gz emd_12607_half_map_2.map.gz emd_12607_half_map_2.map.gz | 194 MB 194 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12607 http://ftp.pdbj.org/pub/emdb/structures/EMD-12607 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12607 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12607 | HTTPS FTP |
-Validation report
| Summary document |  emd_12607_validation.pdf.gz emd_12607_validation.pdf.gz | 986.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_12607_full_validation.pdf.gz emd_12607_full_validation.pdf.gz | 986.2 KB | Display | |
| Data in XML |  emd_12607_validation.xml.gz emd_12607_validation.xml.gz | 21.7 KB | Display | |
| Data in CIF |  emd_12607_validation.cif.gz emd_12607_validation.cif.gz | 28.7 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12607 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12607 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12607 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12607 | HTTPS FTP |
-Related structure data
| Related structure data |  7nvnMC  7nvlC  7nvmC  7nvoC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_12607.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12607.map.gz / Format: CCP4 / Size: 244.1 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 1.06 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_12607_msk_1.map emd_12607_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
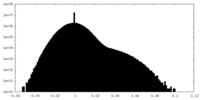
| Density Histograms |
-Half map: #2
| File | emd_12607_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
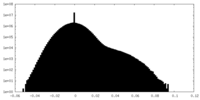
| Density Histograms |
-Half map: #1
| File | emd_12607_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
+Entire : Human type II chaperonin TRiC/CCT complex with nanobody Nb18 and ...
+Supramolecule #1: Human type II chaperonin TRiC/CCT complex with nanobody Nb18 and ...
+Supramolecule #2: Human type II chaperonin TRiC/CCT complex
+Supramolecule #3: Nanobody
+Supramolecule #4: T-complex protein 1 subunit epsilon
+Macromolecule #1: T-complex protein 1 subunit alpha
+Macromolecule #2: T-complex protein 1 subunit beta
+Macromolecule #3: T-complex protein 1 subunit delta
+Macromolecule #4: T-complex protein 1 subunit epsilon
+Macromolecule #5: T-complex protein 1 subunit gamma
+Macromolecule #6: T-complex protein 1 subunit eta
+Macromolecule #7: Nanobody
+Macromolecule #8: T-complex protein 1 subunit theta
+Macromolecule #9: T-complex protein 1 subunit zeta
+Macromolecule #10: Tubulin beta-2A chain
+Macromolecule #11: ADENOSINE-5'-DIPHOSPHATE
+Macromolecule #12: MAGNESIUM ION
+Macromolecule #13: ALUMINUM FLUORIDE
+Macromolecule #14: water
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Buffer | pH: 7.5 |
|---|---|
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 43.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller



















































 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)