+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-12125 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | human Teneurin4 Mut C2 | |||||||||
 Map data Map data | Teneurin4 mut C2 | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | Synaptic cell adhesion / MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology informationcardiac cell fate specification / central nervous system myelin formation / positive regulation of myelination / synaptic membrane adhesion / positive regulation of gastrulation / gastrulation with mouth forming second / regulation of myelination / cardiac muscle cell proliferation / positive regulation of oligodendrocyte differentiation / neuron development ...cardiac cell fate specification / central nervous system myelin formation / positive regulation of myelination / synaptic membrane adhesion / positive regulation of gastrulation / gastrulation with mouth forming second / regulation of myelination / cardiac muscle cell proliferation / positive regulation of oligodendrocyte differentiation / neuron development / cell adhesion molecule binding / neuron projection / protein heterodimerization activity / glutamatergic synapse / signal transduction / protein homodimerization activity / nucleus / plasma membrane / cytoplasm Similarity search - Function | |||||||||
| Biological species |  Homo sapiens (human) Homo sapiens (human) | |||||||||
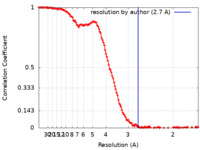
| Method | single particle reconstruction / cryo EM / Resolution: 2.7 Å | |||||||||
 Authors Authors | Meijer DH / Janssen BJC | |||||||||
| Funding support |  Netherlands, 2 items Netherlands, 2 items
| |||||||||
 Citation Citation |  Journal: EMBO J / Year: 2022 Journal: EMBO J / Year: 2022Title: Teneurin4 dimer structures reveal a calcium-stabilized compact conformation supporting homomeric trans-interactions. Authors: Dimphna H Meijer / Cátia P Frias / J Wouter Beugelink / Yanthi N Deurloo / Bert J C Janssen /  Abstract: Establishment of correct synaptic connections is a crucial step during neural circuitry formation. The Teneurin family of neuronal transmembrane proteins promotes cell-cell adhesion via homophilic ...Establishment of correct synaptic connections is a crucial step during neural circuitry formation. The Teneurin family of neuronal transmembrane proteins promotes cell-cell adhesion via homophilic and heterophilic interactions, and is required for synaptic partner matching in the visual and hippocampal systems in vertebrates. It remains unclear how individual Teneurins form macromolecular cis- and trans-synaptic protein complexes. Here, we present a 2.7 Å cryo-EM structure of the dimeric ectodomain of human Teneurin4. The structure reveals a compact conformation of the dimer, stabilized by interactions mediated by the C-rich, YD-shell, and ABD domains. A 1.5 Å crystal structure of the C-rich domain shows three conserved calcium binding sites, and thermal unfolding assays and SAXS-based rigid-body modeling demonstrate that the compactness and stability of Teneurin4 dimers are calcium-dependent. Teneurin4 dimers form a more extended conformation in conditions that lack calcium. Cellular assays reveal that the compact cis-dimer is compatible with homomeric trans-interactions. Together, these findings support a role for teneurins as a scaffold for macromolecular complex assembly and the establishment of cis- and trans-synaptic interactions to construct functional neuronal circuits. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_12125.map.gz emd_12125.map.gz | 110.4 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-12125-v30.xml emd-12125-v30.xml emd-12125.xml emd-12125.xml | 13 KB 13 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_12125_fsc.xml emd_12125_fsc.xml | 16.5 KB | Display |  FSC data file FSC data file |
| Images |  emd_12125.png emd_12125.png | 199.4 KB | ||
| Masks |  emd_12125_msk_1.map emd_12125_msk_1.map | 178 MB |  Mask map Mask map | |
| Filedesc metadata |  emd-12125.cif.gz emd-12125.cif.gz | 6.5 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-12125 http://ftp.pdbj.org/pub/emdb/structures/EMD-12125 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12125 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-12125 | HTTPS FTP |
-Validation report
| Summary document |  emd_12125_validation.pdf.gz emd_12125_validation.pdf.gz | 494.4 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_12125_full_validation.pdf.gz emd_12125_full_validation.pdf.gz | 494 KB | Display | |
| Data in XML |  emd_12125_validation.xml.gz emd_12125_validation.xml.gz | 13.2 KB | Display | |
| Data in CIF |  emd_12125_validation.cif.gz emd_12125_validation.cif.gz | 17.8 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12125 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12125 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12125 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-12125 | HTTPS FTP |
-Related structure data
| Related structure data |  7banMC  7bamC  7baoC  7plpC C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_12125.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_12125.map.gz / Format: CCP4 / Size: 178 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Teneurin4 mut C2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 0.842 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
-Mask #1
| File |  emd_12125_msk_1.map emd_12125_msk_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : human Teneurin4 wt C2 ectodomain
| Entire | Name: human Teneurin4 wt C2 ectodomain |
|---|---|
| Components |
|
-Supramolecule #1: human Teneurin4 wt C2 ectodomain
| Supramolecule | Name: human Teneurin4 wt C2 ectodomain / type: organelle_or_cellular_component / ID: 1 / Parent: 0 / Macromolecule list: #1 |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Teneurin-4
| Macromolecule | Name: Teneurin-4 / type: protein_or_peptide / ID: 1 / Number of copies: 2 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 217.699188 KDa |
| Recombinant expression | Organism:  Homo sapiens (human) Homo sapiens (human) |
| Sequence | String: METACGDSKD NDGDGLVDCM DPDCCLQPLC HINPLCLGSP NPLDIIQETQ VPVSQQNLHS FYDRIKFLVG RDSTHIIPGE NPFDGGHAC VIRGQVMTSD GTPLVGVNIS FVNNPLFGYT ISRQDGSFDL VTNGGISIIL RFERAPFITQ EHTLWLPWDR F FVMETIIM ...String: METACGDSKD NDGDGLVDCM DPDCCLQPLC HINPLCLGSP NPLDIIQETQ VPVSQQNLHS FYDRIKFLVG RDSTHIIPGE NPFDGGHAC VIRGQVMTSD GTPLVGVNIS FVNNPLFGYT ISRQDGSFDL VTNGGISIIL RFERAPFITQ EHTLWLPWDR F FVMETIIM RHEENEIPSC DLSNFARPNP VVSPSPLTSF ASSCAEKGPI VPEIQALQEE ISISGCKMRL SYLSSRTPGY KS VLRISLT HPTIPFNLMK VHLMVAVEGR LFRKWFAAAP DLSYYFIWDK TDVYNQKVFG LSEAFVSVGY EYESCPDLIL WEK RTTVLQ GYEIDASKLG GWSLDKHHAL NIQSGILHKG NGENQFVSQQ PPVIGSIMGN GRRRSISCPS CNGLADGNKL LAPV ALTCG SDGSLYVGDF NYIRRIFPSG NVTNILELRN KDFRHSHSPA HKYYLATDPM SGAVFLSDSN SRRVFKIKST VVVKD LVKN SEVVAGTGDQ CLPFDDTRCG DGGKATEATL TNPRGITVDK FGLIYFVDGT MIRRIDQNGI ISTLLGSNDL TSARPL SCD SVMDISQVHL EWPTDLAINP MDNSLYVLDN NVVLQISENH QVRIVAGRPM HCQVPGIDHF LLSKVAIHAT LESATAL AV SHNGVLYIAE TDEKKINRIR QVTTSGEISL VAGAPSGCDC KNDANCDCFS GDDGYAKDAK LNTPSSLAVC ADGELYVA D LGNIRIRFIR KNKPFLNTQN MYELSSPIDQ ELYLFDTTGK HLYTQSLPTG DYLYNFTYTG DGDITLITDN NGNMVNVRR DSTGMPLWLV VPDGQVYWVT MGTNSALKSV TTQGHELAMM TYHGNSGLLA TKSNENGWTT FYEYDSFGRL TNVTFPTGQV SSFRSDTDS SVHVQVETSS KDDVTITTNL SASGAFYTLL QDQVRNSYYI GADGSLRLLL ANGMEVALQT EPHLLAGTVN P TVGKRNVT LPIDNGLNLV EWRQRKEQAR GQVTVFGRRL RVHNRNLLSL DFDRVTRTEK IYDDHRKFTL RILYDQAGRP SL WSPSSRL NGVNVTYSPG GYIAGIQRGI MSERMEYDQA GRITSRIFAD GKTWSYTYLE KSMVLLLHSQ RQYIFEFDKN DRL SSVTMP NVARQTLETI RSVGYYRNIY QPPEGNASVI QDFTEDGHLL HTFYLGTGRR VIYKYGKLSK LAETLYDTTK VSFT YDETA GMLKTINLQN EGFTCTIRYR QIGPLIDRQI FRFTEEGMVN ARFDYNYDNS FRVTSMQAVI NETPLPIDLY RYDDV SGKT EQFGKFGVIY YDINQIITTA VMTHTKHFDA YGRMKEVQYE IFRSLMYWMT VQYDNMGRVV KKELKVGPYA NTTRYS YEY DADGQLQTVS INDKPLWRYS YDLNGNLHLL SPGNSARLTP LRYDIRDRIT RLGDVQYKMD EDGFLRQRGG DIFEYNS AG LLIKAYNRAG SWSVRYRYDG LGRRVSSKSS HSHHLQFFYA DLTNPTKVTH LYNHSSSEIT SLYYDLQGHL FAMELSSG D EFYIACDNIG TPLAVFSGTG LMIKQILYTA YGEIYMDTNP NFQIIIGYHG GLYDPLTKLV HMGRRDYDVL AGRWTSPDH ELWKHLSSSN VMPFNLYMFK NNNPISNSQD IKCFMTDVNS WLLTFGFQLH NVIPGYPKPD MDAMEPSYEL IHTQMKTQEW DNSKSILGV QCEVQKQLKA FVTLERFDQL YGSTITSCQQ APKTKKFASS GSVFGKGVKF ALKDGRVTTD IICVANEDGR R VAAILNHA HYLENLHFTI DGVDTHYFVK PGPSEGDLAI LGLSGGRRTL ENGVNVTVSQ INTVLNGRTR RYTDIQLQYG AL CLNTRYG TTLDEEKARV LELARQRAVR QAWAREQQRL REGEEGLRAW TEGEKQQVLS TGRVQGYDGF FVISVEQYPE LSD SANNIH FMRQSE UniProtKB: Teneurin-4 |
-Macromolecule #3: 2-acetamido-2-deoxy-beta-D-glucopyranose
| Macromolecule | Name: 2-acetamido-2-deoxy-beta-D-glucopyranose / type: ligand / ID: 3 / Number of copies: 14 / Formula: NAG |
|---|---|
| Molecular weight | Theoretical: 221.208 Da |
| Chemical component information |  ChemComp-NAG: |
-Macromolecule #4: CALCIUM ION
| Macromolecule | Name: CALCIUM ION / type: ligand / ID: 4 / Number of copies: 6 / Formula: CA |
|---|---|
| Molecular weight | Theoretical: 40.078 Da |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 0.075 mg/mL |
|---|---|
| Buffer | pH: 7.8 |
| Vitrification | Cryogen name: ETHANE |
- Electron microscopy
Electron microscopy
| Microscope | TFS KRIOS |
|---|---|
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Average electron dose: 50.6 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: OTHER / Imaging mode: BRIGHT FIELD |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller























 Z
Z Y
Y X
X