+ Open data
Open data
- Basic information
Basic information
| Entry | Database: EMDB / ID: EMD-10929 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | 3D structure of bacteriophage phAPEC6 tail | |||||||||
 Map data Map data | Entire phAPEC6 tail. Resolution 25 A | |||||||||
 Sample Sample |
| |||||||||
| Biological species |  | |||||||||
| Method | single particle reconstruction / cryo EM / Resolution: 25.0 Å | |||||||||
 Authors Authors | Wagemans J / Tsonos J / Holtappels D / Fortuna K / Hernalsteens JP / De Greve H / Estrozi LF / Bacia-Verloop M / Moriscot C / Noben JP ...Wagemans J / Tsonos J / Holtappels D / Fortuna K / Hernalsteens JP / De Greve H / Estrozi LF / Bacia-Verloop M / Moriscot C / Noben JP / Schoehn G / Lavigne R | |||||||||
 Citation Citation |  Journal: Int J Mol Sci / Year: 2020 Journal: Int J Mol Sci / Year: 2020Title: Structural Analysis of Jumbo Coliphage phAPEC6. Authors: Jeroen Wagemans / Jessica Tsonos / Dominique Holtappels / Kiandro Fortuna / Jean-Pierre Hernalsteens / Henri De Greve / Leandro F Estrozi / Maria Bacia-Verloop / Christine Moriscot / Jean- ...Authors: Jeroen Wagemans / Jessica Tsonos / Dominique Holtappels / Kiandro Fortuna / Jean-Pierre Hernalsteens / Henri De Greve / Leandro F Estrozi / Maria Bacia-Verloop / Christine Moriscot / Jean-Paul Noben / Guy Schoehn / Rob Lavigne /   Abstract: The phAPEC6 genome encodes 551 predicted gene products, with the vast majority (83%) of unknown function. Of these, 62 have been identified as virion-associated proteins by mass spectrometry (ESI- ...The phAPEC6 genome encodes 551 predicted gene products, with the vast majority (83%) of unknown function. Of these, 62 have been identified as virion-associated proteins by mass spectrometry (ESI-MS/MS), including the major capsid protein (Gp225; present in 1620 copies), which shows a HK97 capsid protein-based fold. Cryo-electron microscopy experiments showed that the 350-kbp DNA molecule of virus phAPEC6 is packaged in at least 15 concentric layers in the phage capsid. A capsid inner body rod is also present, measuring about 91 nm by 18 nm and oriented along the portal axis. In the phAPEC6 contractile tail, 25 hexameric stacked rings can be distinguished, built of the identified tail sheath protein (Gp277). Cryo-EM reconstruction reveals the base of the unique hairy fibers observed during an initial transmission electron microscopy (TEM) analysis. These very unusual filaments are ordered at three annular positions along the contractile sheath, as well as around the capsid, and may be involved in host interaction. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Movie |
 Movie viewer Movie viewer |
|---|---|
| Structure viewer | EM map:  SurfView SurfView Molmil Molmil Jmol/JSmol Jmol/JSmol |
| Supplemental images |
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_10929.map.gz emd_10929.map.gz | 244.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-10929-v30.xml emd-10929-v30.xml emd-10929.xml emd-10929.xml | 10.2 KB 10.2 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_10929.png emd_10929.png | 33.3 KB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-10929 http://ftp.pdbj.org/pub/emdb/structures/EMD-10929 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10929 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-10929 | HTTPS FTP |
-Validation report
| Summary document |  emd_10929_validation.pdf.gz emd_10929_validation.pdf.gz | 272.7 KB | Display |  EMDB validaton report EMDB validaton report |
|---|---|---|---|---|
| Full document |  emd_10929_full_validation.pdf.gz emd_10929_full_validation.pdf.gz | 271.8 KB | Display | |
| Data in XML |  emd_10929_validation.xml.gz emd_10929_validation.xml.gz | 7.4 KB | Display | |
| Arichive directory |  https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10929 https://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10929 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10929 ftp://ftp.pdbj.org/pub/emdb/validation_reports/EMD-10929 | HTTPS FTP |
-Related structure data
| Related structure data | C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|
- Map
Map
| File |  Download / File: emd_10929.map.gz / Format: CCP4 / Size: 292.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_10929.map.gz / Format: CCP4 / Size: 292.8 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Entire phAPEC6 tail. Resolution 25 A | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Projections & slices | Image control
Images are generated by Spider. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Voxel size | X=Y=Z: 4.52 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
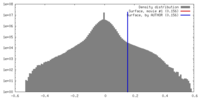
| Density |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Details | EMDB XML:
CCP4 map header:
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
-Supplemental data
- Sample components
Sample components
-Entire : Escherichia coli
| Entire | Name:  |
|---|---|
| Components |
|
-Supramolecule #1: Escherichia coli
| Supramolecule | Name: Escherichia coli / type: virus / ID: 1 / Parent: 0 / NCBI-ID: 562 / Sci species name: Escherichia coli / Virus type: VIRION / Virus isolate: OTHER / Virus enveloped: No / Virus empty: No |
|---|---|
| Host (natural) | Organism: E coli |
-Experimental details
-Structure determination
| Method | cryo EM |
|---|---|
 Processing Processing | single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Grid | Model: Quantifoil R2/1 / Material: COPPER / Mesh: 400 / Support film - Material: CARBON / Support film - topology: HOLEY ARRAY / Pretreatment - Type: GLOW DISCHARGE |
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Instrument: FEI VITROBOT MARK IV / Details: Force 1, 2s blotting time. |
- Electron microscopy
Electron microscopy
| Microscope | FEI POLARA 300 |
|---|---|
| Image recording | Film or detector model: KODAK SO-163 FILM / Number grids imaged: 1 / Number real images: 100 / Average electron dose: 20.0 e/Å2 |
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD / Nominal magnification: 31000 |
| Sample stage | Specimen holder model: OTHER / Cooling holder cryogen: NITROGEN |
| Experimental equipment |  Model: Tecnai Polara / Image courtesy: FEI Company |
 Movie
Movie Controller
Controller






 Z (Sec.)
Z (Sec.) Y (Row.)
Y (Row.) X (Col.)
X (Col.)