[English] 日本語
 Yorodumi
Yorodumi- PDB-8w9w: The cryo-EM structure of human sphingomyelin synthase-related pro... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8w9w | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
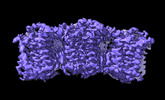
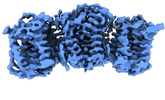
| Title | The cryo-EM structure of human sphingomyelin synthase-related protein in complex with ceramide/phosphoethanolamine | |||||||||
 Components Components | Sphingomyelin synthase-related protein 1 | |||||||||
 Keywords Keywords |  MEMBRANE PROTEIN / MEMBRANE PROTEIN /  synthase / synthase /  sphingomyelin / CPE / sphingomyelin / CPE /  lipid metabolism lipid metabolism | |||||||||
| Function / homology |  Function and homology information Function and homology informationceramide phosphoethanolamine biosynthetic process /  ceramide phosphoethanolamine synthase activity / ceramide phosphoethanolamine synthase activity /  sphingomyelin synthase activity / sphingomyelin synthase activity /  ceramide cholinephosphotransferase activity / regulation of ceramide biosynthetic process / ceramide cholinephosphotransferase activity / regulation of ceramide biosynthetic process /  Transferases; Transferring phosphorus-containing groups; Transferases for other substituted phosphate groups / sphingomyelin biosynthetic process / sphingolipid biosynthetic process / Sphingolipid de novo biosynthesis / ceramide biosynthetic process ...ceramide phosphoethanolamine biosynthetic process / Transferases; Transferring phosphorus-containing groups; Transferases for other substituted phosphate groups / sphingomyelin biosynthetic process / sphingolipid biosynthetic process / Sphingolipid de novo biosynthesis / ceramide biosynthetic process ...ceramide phosphoethanolamine biosynthetic process /  ceramide phosphoethanolamine synthase activity / ceramide phosphoethanolamine synthase activity /  sphingomyelin synthase activity / sphingomyelin synthase activity /  ceramide cholinephosphotransferase activity / regulation of ceramide biosynthetic process / ceramide cholinephosphotransferase activity / regulation of ceramide biosynthetic process /  Transferases; Transferring phosphorus-containing groups; Transferases for other substituted phosphate groups / sphingomyelin biosynthetic process / sphingolipid biosynthetic process / Sphingolipid de novo biosynthesis / ceramide biosynthetic process / endoplasmic reticulum membrane / Transferases; Transferring phosphorus-containing groups; Transferases for other substituted phosphate groups / sphingomyelin biosynthetic process / sphingolipid biosynthetic process / Sphingolipid de novo biosynthesis / ceramide biosynthetic process / endoplasmic reticulum membrane /  endoplasmic reticulum / endoplasmic reticulum /  membrane / membrane /  cytosol cytosolSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.74 Å cryo EM / Resolution: 3.74 Å | |||||||||
 Authors Authors | Hu, K. / Zhang, Q. / Chen, Y. / Yao, D. / Zhou, L. / Cao, Y. | |||||||||
| Funding support |  China, 2items China, 2items
| |||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2024 Journal: Nat Struct Mol Biol / Year: 2024Title: Cryo-EM structure of human sphingomyelin synthase and its mechanistic implications for sphingomyelin synthesis. Authors: Kexin Hu / Qing Zhang / Yang Chen / Jintong Yang / Ying Xia / Bing Rao / Shaobai Li / Yafeng Shen / Mi Cao / Hongliang Lu / An Qin / Xian-Cheng Jiang / Deqiang Yao / Jie Zhao / Lu Zhou / Yu Cao /   Abstract: Sphingomyelin (SM) has key roles in modulating mammalian membrane properties and serves as an important pool for bioactive molecules. SM biosynthesis is mediated by the sphingomyelin synthase (SMS) ...Sphingomyelin (SM) has key roles in modulating mammalian membrane properties and serves as an important pool for bioactive molecules. SM biosynthesis is mediated by the sphingomyelin synthase (SMS) family, comprising SMS1, SMS2 and SMS-related (SMSr) members. Although SMS1 and SMS2 exhibit SMS activity, SMSr possesses ceramide phosphoethanolamine synthase activity. Here we determined the cryo-electron microscopic structures of human SMSr in complexes with ceramide, diacylglycerol/phosphoethanolamine and ceramide/phosphoethanolamine (CPE). The structures revealed a hexameric arrangement with a reaction chamber located between the transmembrane helices. Within this structure, a catalytic pentad E-H/D-H-D was identified, situated at the interface between the lipophilic and hydrophilic segments of the reaction chamber. Additionally, the study unveiled the two-step synthesis process catalyzed by SMSr, involving PE-PLC (phosphatidylethanolamine-phospholipase C) hydrolysis and the subsequent transfer of the phosphoethanolamine moiety to ceramide. This research provides insights into the catalytic mechanism of SMSr and expands our understanding of sphingolipid metabolism. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8w9w.cif.gz 8w9w.cif.gz | 291 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8w9w.ent.gz pdb8w9w.ent.gz | 242.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8w9w.json.gz 8w9w.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/w9/8w9w https://data.pdbj.org/pub/pdb/validation_reports/w9/8w9w ftp://data.pdbj.org/pub/pdb/validation_reports/w9/8w9w ftp://data.pdbj.org/pub/pdb/validation_reports/w9/8w9w | HTTPS FTP |
|---|
-Related structure data
| Related structure data | 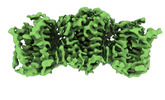 37383MC  8ijqC  8ijrC  8w9yC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
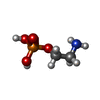
| #1: Protein | Mass: 31056.529 Da / Num. of mol.: 6 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: SAMD8 / Production host: Homo sapiens (human) / Gene: SAMD8 / Production host:   Homo sapiens (human) / References: UniProt: Q96LT4, Homo sapiens (human) / References: UniProt: Q96LT4,  sphingomyelin synthase sphingomyelin synthase#2: Chemical | ChemComp-UJO / ~{ Mass: 467.768 Da / Num. of mol.: 6 / Source method: obtained synthetically / Formula: C29H57NO3 / Feature type: SUBJECT OF INVESTIGATION #3: Chemical | ChemComp-OPE /  Phosphorylethanolamine PhosphorylethanolamineHas ligand of interest | Y | |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: The cryo-EM structure of human sphingomyelin synthase-related protein in complex with ceramide/phosphoethanolamine Type: COMPLEX / Entity ID: #1 / Source: RECOMBINANT |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Buffer solution | pH: 7.5 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES |
Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: SPOT SCAN FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: SPOT SCAN |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 2600 nm / Nominal defocus min: 1000 nm Bright-field microscopy / Nominal defocus max: 2600 nm / Nominal defocus min: 1000 nm |
| Image recording | Electron dose: 50 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) |
- Processing
Processing
| EM software | Name: PHENIX / Version: 1.20.1_4487: / Category: model refinement | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
3D reconstruction | Resolution: 3.74 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 700891 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller



 PDBj
PDBj