+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8u3z | ||||||
|---|---|---|---|---|---|---|---|
| Title | Model of TTLL6 bound to microtubule from composite map | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  LIGASE / LIGASE /  tubulin / post-translational modifications / tubulin / post-translational modifications /  microtubules / Tubulin Tyrosine Ligase Like family enzymes microtubules / Tubulin Tyrosine Ligase Like family enzymes | ||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of cilium movement / protein-glutamic acid ligase activity / tubulin-glutamic acid ligase activity / Carboxyterminal post-translational modifications of tubulin / protein polyglutamylation / regulation of cilium beat frequency involved in ciliary motility / 9+0 non-motile cilium / microtubule severing /  Ligases; Forming carbon-nitrogen bonds; Acid-amino-acid ligases (peptide synthases) / odontoblast differentiation ...positive regulation of cilium movement / protein-glutamic acid ligase activity / tubulin-glutamic acid ligase activity / Carboxyterminal post-translational modifications of tubulin / protein polyglutamylation / regulation of cilium beat frequency involved in ciliary motility / 9+0 non-motile cilium / microtubule severing / Ligases; Forming carbon-nitrogen bonds; Acid-amino-acid ligases (peptide synthases) / odontoblast differentiation ...positive regulation of cilium movement / protein-glutamic acid ligase activity / tubulin-glutamic acid ligase activity / Carboxyterminal post-translational modifications of tubulin / protein polyglutamylation / regulation of cilium beat frequency involved in ciliary motility / 9+0 non-motile cilium / microtubule severing /  Ligases; Forming carbon-nitrogen bonds; Acid-amino-acid ligases (peptide synthases) / odontoblast differentiation / Post-chaperonin tubulin folding pathway / Carboxyterminal post-translational modifications of tubulin / Microtubule-dependent trafficking of connexons from Golgi to the plasma membrane / Ligases; Forming carbon-nitrogen bonds; Acid-amino-acid ligases (peptide synthases) / odontoblast differentiation / Post-chaperonin tubulin folding pathway / Carboxyterminal post-translational modifications of tubulin / Microtubule-dependent trafficking of connexons from Golgi to the plasma membrane /  Cilium Assembly / Sealing of the nuclear envelope (NE) by ESCRT-III / Cilium Assembly / Sealing of the nuclear envelope (NE) by ESCRT-III /  Intraflagellar transport / cytoskeleton-dependent intracellular transport / Formation of tubulin folding intermediates by CCT/TriC / Intraflagellar transport / cytoskeleton-dependent intracellular transport / Formation of tubulin folding intermediates by CCT/TriC /  Gap junction assembly / COPI-independent Golgi-to-ER retrograde traffic / natural killer cell mediated cytotoxicity / microtubule bundle formation / Assembly and cell surface presentation of NMDA receptors / Kinesins / GTPase activating protein binding / COPI-dependent Golgi-to-ER retrograde traffic / Gap junction assembly / COPI-independent Golgi-to-ER retrograde traffic / natural killer cell mediated cytotoxicity / microtubule bundle formation / Assembly and cell surface presentation of NMDA receptors / Kinesins / GTPase activating protein binding / COPI-dependent Golgi-to-ER retrograde traffic /  intercellular bridge / regulation of synapse organization / nuclear envelope lumen / cytoplasmic microtubule / Recycling pathway of L1 / MHC class I protein binding / RHOH GTPase cycle / RHO GTPases activate IQGAPs / spindle assembly / cellular response to interleukin-4 / microtubule-based process / Hedgehog 'off' state / COPI-mediated anterograde transport / Activation of AMPK downstream of NMDARs / Mitotic Prometaphase / EML4 and NUDC in mitotic spindle formation / Loss of Nlp from mitotic centrosomes / Loss of proteins required for interphase microtubule organization from the centrosome / Recruitment of mitotic centrosome proteins and complexes / Resolution of Sister Chromatid Cohesion / Recruitment of NuMA to mitotic centrosomes / Anchoring of the basal body to the plasma membrane / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / MHC class II antigen presentation / intercellular bridge / regulation of synapse organization / nuclear envelope lumen / cytoplasmic microtubule / Recycling pathway of L1 / MHC class I protein binding / RHOH GTPase cycle / RHO GTPases activate IQGAPs / spindle assembly / cellular response to interleukin-4 / microtubule-based process / Hedgehog 'off' state / COPI-mediated anterograde transport / Activation of AMPK downstream of NMDARs / Mitotic Prometaphase / EML4 and NUDC in mitotic spindle formation / Loss of Nlp from mitotic centrosomes / Loss of proteins required for interphase microtubule organization from the centrosome / Recruitment of mitotic centrosome proteins and complexes / Resolution of Sister Chromatid Cohesion / Recruitment of NuMA to mitotic centrosomes / Anchoring of the basal body to the plasma membrane / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / MHC class II antigen presentation /  tubulin binding / AURKA Activation by TPX2 / ciliary basal body / Translocation of SLC2A4 (GLUT4) to the plasma membrane / RHO GTPases Activate Formins / tubulin binding / AURKA Activation by TPX2 / ciliary basal body / Translocation of SLC2A4 (GLUT4) to the plasma membrane / RHO GTPases Activate Formins /  Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / PKR-mediated signaling / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / PKR-mediated signaling /  cilium / cilium /  mitotic spindle / structural constituent of cytoskeleton / microtubule cytoskeleton organization / Aggrephagy / cytoplasmic ribonucleoprotein granule / HCMV Early Events / Separation of Sister Chromatids / The role of GTSE1 in G2/M progression after G2 checkpoint / microtubule cytoskeleton / mitotic spindle / structural constituent of cytoskeleton / microtubule cytoskeleton organization / Aggrephagy / cytoplasmic ribonucleoprotein granule / HCMV Early Events / Separation of Sister Chromatids / The role of GTSE1 in G2/M progression after G2 checkpoint / microtubule cytoskeleton /  Regulation of PLK1 Activity at G2/M Transition / azurophil granule lumen / Regulation of PLK1 Activity at G2/M Transition / azurophil granule lumen /  double-stranded RNA binding / mitotic cell cycle / double-stranded RNA binding / mitotic cell cycle /  cell body / cell body /  microtubule / Potential therapeutics for SARS / microtubule / Potential therapeutics for SARS /  cytoskeleton / cytoskeleton /  membrane raft / protein domain specific binding / membrane raft / protein domain specific binding /  cell division / cell division /  GTPase activity / GTPase activity /  ubiquitin protein ligase binding / Neutrophil degranulation / protein-containing complex binding / GTP binding / structural molecule activity / protein-containing complex / extracellular exosome / extracellular region / ubiquitin protein ligase binding / Neutrophil degranulation / protein-containing complex binding / GTP binding / structural molecule activity / protein-containing complex / extracellular exosome / extracellular region /  ATP binding / ATP binding /  metal ion binding / metal ion binding /  nucleus / nucleus /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Mus musculus (house mouse) Mus musculus (house mouse)  Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.6 Å cryo EM / Resolution: 3.6 Å | ||||||
 Authors Authors | Mahalingan, K.K. / Grotjahn, D. / Li, Y. / Lander, G.C. / Zehr, E.A. / Roll-Mecak, A. | ||||||
| Funding support |  United States, 1items United States, 1items
| ||||||
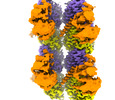
 Citation Citation |  Journal: Nat Chem Biol / Year: 2024 Journal: Nat Chem Biol / Year: 2024Title: Structural basis for α-tubulin-specific and modification state-dependent glutamylation. Authors: Kishore K Mahalingan / Danielle A Grotjahn / Yan Li / Gabriel C Lander / Elena A Zehr / Antonina Roll-Mecak /  Abstract: Microtubules have spatiotemporally complex posttranslational modification patterns. Tubulin tyrosine ligase-like (TTLL) enzymes introduce the most prevalent modifications on α-tubulin and β-tubulin. ...Microtubules have spatiotemporally complex posttranslational modification patterns. Tubulin tyrosine ligase-like (TTLL) enzymes introduce the most prevalent modifications on α-tubulin and β-tubulin. How TTLLs specialize for specific substrate recognition and ultimately modification-pattern generation is largely unknown. TTLL6, a glutamylase implicated in ciliopathies, preferentially modifies tubulin α-tails in microtubules. Cryo-electron microscopy, kinetic analysis and single-molecule biochemistry reveal an unprecedented quadrivalent recognition that ensures simultaneous readout of microtubule geometry and posttranslational modification status. By binding to a β-tubulin subunit, TTLL6 modifies the α-tail of the longitudinally adjacent tubulin dimer. Spanning two tubulin dimers along and across protofilaments (PFs) ensures fidelity of recognition of both the α-tail and the microtubule. Moreover, TTLL6 reads out and is stimulated by glutamylation of the β-tail of the laterally adjacent tubulin dimer, mediating crosstalk between α-tail and β-tail. This positive feedback loop can generate localized microtubule glutamylation patterns. Our work uncovers general principles that generate tubulin chemical and topographic complexity. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8u3z.cif.gz 8u3z.cif.gz | 425.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8u3z.ent.gz pdb8u3z.ent.gz | 338.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8u3z.json.gz 8u3z.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/u3/8u3z https://data.pdbj.org/pub/pdb/validation_reports/u3/8u3z ftp://data.pdbj.org/pub/pdb/validation_reports/u3/8u3z ftp://data.pdbj.org/pub/pdb/validation_reports/u3/8u3z | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  41090MC  8t42C C: citing same article ( M: map data used to model this data |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
| Experimental dataset #1 | Data reference:  10.6019/EMPIAR-11798 / Data set type: EMPIAR 10.6019/EMPIAR-11798 / Data set type: EMPIARDetails: Cryo-EM micrographs of human alpha1B/BetaI+BetaIVb microtubules bound to GMPCPP decorated with TTLL6 Metadata reference: 10.6019/EMPIAR-11798 |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Protein , 3 types, 5 molecules AFBEN
| #1: Protein | Mass: 50204.445 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Homo sapiens (human) / Cell line: tsA201 / Organ: kidney Homo sapiens (human) / Cell line: tsA201 / Organ: kidney / Tissue: kidney / Tissue: kidney / References: UniProt: P68363 / References: UniProt: P68363#2: Protein | Mass: 49717.629 Da / Num. of mol.: 2 / Source method: isolated from a natural source / Source: (natural)   Homo sapiens (human) / Cell line: tsA201 / Organ: kidney Homo sapiens (human) / Cell line: tsA201 / Organ: kidney / Tissue: kidney / Tissue: kidney / References: UniProt: P07437 / References: UniProt: P07437#3: Protein | | Mass: 94601.188 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Mus musculus (house mouse) / Gene: Ttll6 / Plasmid: P7XNH3 / Production host: Mus musculus (house mouse) / Gene: Ttll6 / Plasmid: P7XNH3 / Production host:   Escherichia coli (E. coli) / Strain (production host): Arctic Express DE3 Escherichia coli (E. coli) / Strain (production host): Arctic Express DE3References: UniProt: A4Q9E8,  Ligases; Forming carbon-nitrogen bonds; Acid-amino-acid ligases (peptide synthases) Ligases; Forming carbon-nitrogen bonds; Acid-amino-acid ligases (peptide synthases) |
|---|
-Non-polymers , 4 types, 10 molecules 






| #4: Chemical |  Guanosine triphosphate Guanosine triphosphate#5: Chemical | ChemComp-MG / #6: Chemical | #7: Chemical | ChemComp-ATP / |  Adenosine triphosphate Adenosine triphosphate |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: FILAMENT / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight |
| |||||||||||||||||||||||||||||||||||
| Source (natural) |
| |||||||||||||||||||||||||||||||||||
| Source (recombinant) | Organism:   Escherichia coli (E. coli) / Strain: Arctic Express DE3 / Plasmid Escherichia coli (E. coli) / Strain: Arctic Express DE3 / Plasmid : P7XNH3 : P7XNH3 | |||||||||||||||||||||||||||||||||||
| Buffer solution | pH: 7 | |||||||||||||||||||||||||||||||||||
| Buffer component |
| |||||||||||||||||||||||||||||||||||
| Specimen | Conc.: 0.1 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES / Details: TTLL6 decorated microtubules heterogeneously : YES / Details: TTLL6 decorated microtubules heterogeneously | |||||||||||||||||||||||||||||||||||
| Specimen support | Grid material: GOLD / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R1.2/1.3 | |||||||||||||||||||||||||||||||||||
Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 303 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TALOS ARCTICA |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal magnification: 36000 X / Nominal defocus max: 2000 nm / Nominal defocus min: 1000 nm / Cs Bright-field microscopy / Nominal magnification: 36000 X / Nominal defocus max: 2000 nm / Nominal defocus min: 1000 nm / Cs : 2.7 mm / Alignment procedure: COMA FREE : 2.7 mm / Alignment procedure: COMA FREE |
| Image recording | Average exposure time: 9.75 sec. / Electron dose: 42 e/Å2 / Detector mode: COUNTING / Film or detector model: GATAN K2 SUMMIT (4k x 4k) |
| Image scans | Movie frames/image: 39 / Used frames/image: 1-39 |
- Processing
Processing
| EM software |
| ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 59055 Details: Due to highly heterogeneous TTLL decoration microtubules were manually selected. 59044 overlapping segments with a box size of 568 pixels were extracted every 82 A | ||||||||||||||||||||||||||||
| Symmetry | Point symmetry : C1 (asymmetric) : C1 (asymmetric) | ||||||||||||||||||||||||||||
3D reconstruction | Resolution: 3.6 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 392289 / Algorithm: FOURIER SPACE / Num. of class averages: 1 / Symmetry type: POINT | ||||||||||||||||||||||||||||
| Atomic model building | Protocol: FLEXIBLE FIT / Space: REAL / Target criteria: cross-correlation | ||||||||||||||||||||||||||||
| Atomic model building | 3D fitting-ID: 1 / Source name: PDB / Type: experimental model
|
 Movie
Movie Controller
Controller





 PDBj
PDBj

































