+ Open data
Open data
- Basic information
Basic information
| Entry |  | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
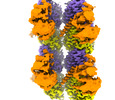
| Title | Composite map of TTLL6 bound to unmodified human microtubule | |||||||||
 Map data Map data | Composite map of TTLL6 bound to unmodified human microtubules. | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords | tubulin post-translational modifications /  microtubules / TTLL / microtubules / TTLL /  LIGASE LIGASE | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of cilium movement / protein-glutamic acid ligase activity / tubulin-glutamic acid ligase activity / Carboxyterminal post-translational modifications of tubulin / protein polyglutamylation / regulation of cilium beat frequency involved in ciliary motility / 9+0 non-motile cilium / microtubule severing /  Ligases; Forming carbon-nitrogen bonds; Acid-amino-acid ligases (peptide synthases) / odontoblast differentiation ...positive regulation of cilium movement / protein-glutamic acid ligase activity / tubulin-glutamic acid ligase activity / Carboxyterminal post-translational modifications of tubulin / protein polyglutamylation / regulation of cilium beat frequency involved in ciliary motility / 9+0 non-motile cilium / microtubule severing / Ligases; Forming carbon-nitrogen bonds; Acid-amino-acid ligases (peptide synthases) / odontoblast differentiation ...positive regulation of cilium movement / protein-glutamic acid ligase activity / tubulin-glutamic acid ligase activity / Carboxyterminal post-translational modifications of tubulin / protein polyglutamylation / regulation of cilium beat frequency involved in ciliary motility / 9+0 non-motile cilium / microtubule severing /  Ligases; Forming carbon-nitrogen bonds; Acid-amino-acid ligases (peptide synthases) / odontoblast differentiation / Post-chaperonin tubulin folding pathway / Carboxyterminal post-translational modifications of tubulin / Microtubule-dependent trafficking of connexons from Golgi to the plasma membrane / Ligases; Forming carbon-nitrogen bonds; Acid-amino-acid ligases (peptide synthases) / odontoblast differentiation / Post-chaperonin tubulin folding pathway / Carboxyterminal post-translational modifications of tubulin / Microtubule-dependent trafficking of connexons from Golgi to the plasma membrane /  Cilium Assembly / Sealing of the nuclear envelope (NE) by ESCRT-III / Cilium Assembly / Sealing of the nuclear envelope (NE) by ESCRT-III /  Intraflagellar transport / cytoskeleton-dependent intracellular transport / Formation of tubulin folding intermediates by CCT/TriC / Intraflagellar transport / cytoskeleton-dependent intracellular transport / Formation of tubulin folding intermediates by CCT/TriC /  Gap junction assembly / COPI-independent Golgi-to-ER retrograde traffic / natural killer cell mediated cytotoxicity / microtubule bundle formation / Assembly and cell surface presentation of NMDA receptors / Kinesins / GTPase activating protein binding / COPI-dependent Golgi-to-ER retrograde traffic / Gap junction assembly / COPI-independent Golgi-to-ER retrograde traffic / natural killer cell mediated cytotoxicity / microtubule bundle formation / Assembly and cell surface presentation of NMDA receptors / Kinesins / GTPase activating protein binding / COPI-dependent Golgi-to-ER retrograde traffic /  intercellular bridge / regulation of synapse organization / nuclear envelope lumen / cytoplasmic microtubule / Recycling pathway of L1 / MHC class I protein binding / RHOH GTPase cycle / RHO GTPases activate IQGAPs / spindle assembly / cellular response to interleukin-4 / microtubule-based process / Hedgehog 'off' state / COPI-mediated anterograde transport / Activation of AMPK downstream of NMDARs / Mitotic Prometaphase / EML4 and NUDC in mitotic spindle formation / Loss of Nlp from mitotic centrosomes / Loss of proteins required for interphase microtubule organization from the centrosome / Recruitment of mitotic centrosome proteins and complexes / Resolution of Sister Chromatid Cohesion / Recruitment of NuMA to mitotic centrosomes / Anchoring of the basal body to the plasma membrane / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / MHC class II antigen presentation / intercellular bridge / regulation of synapse organization / nuclear envelope lumen / cytoplasmic microtubule / Recycling pathway of L1 / MHC class I protein binding / RHOH GTPase cycle / RHO GTPases activate IQGAPs / spindle assembly / cellular response to interleukin-4 / microtubule-based process / Hedgehog 'off' state / COPI-mediated anterograde transport / Activation of AMPK downstream of NMDARs / Mitotic Prometaphase / EML4 and NUDC in mitotic spindle formation / Loss of Nlp from mitotic centrosomes / Loss of proteins required for interphase microtubule organization from the centrosome / Recruitment of mitotic centrosome proteins and complexes / Resolution of Sister Chromatid Cohesion / Recruitment of NuMA to mitotic centrosomes / Anchoring of the basal body to the plasma membrane / HSP90 chaperone cycle for steroid hormone receptors (SHR) in the presence of ligand / MHC class II antigen presentation /  tubulin binding / AURKA Activation by TPX2 / ciliary basal body / Translocation of SLC2A4 (GLUT4) to the plasma membrane / RHO GTPases Activate Formins / tubulin binding / AURKA Activation by TPX2 / ciliary basal body / Translocation of SLC2A4 (GLUT4) to the plasma membrane / RHO GTPases Activate Formins /  Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / PKR-mediated signaling / Hydrolases; Acting on acid anhydrides; Acting on GTP to facilitate cellular and subcellular movement / PKR-mediated signaling /  cilium / cilium /  mitotic spindle / structural constituent of cytoskeleton / microtubule cytoskeleton organization / Aggrephagy / cytoplasmic ribonucleoprotein granule / HCMV Early Events / Separation of Sister Chromatids / The role of GTSE1 in G2/M progression after G2 checkpoint / microtubule cytoskeleton / mitotic spindle / structural constituent of cytoskeleton / microtubule cytoskeleton organization / Aggrephagy / cytoplasmic ribonucleoprotein granule / HCMV Early Events / Separation of Sister Chromatids / The role of GTSE1 in G2/M progression after G2 checkpoint / microtubule cytoskeleton /  Regulation of PLK1 Activity at G2/M Transition / azurophil granule lumen / Regulation of PLK1 Activity at G2/M Transition / azurophil granule lumen /  double-stranded RNA binding / mitotic cell cycle / double-stranded RNA binding / mitotic cell cycle /  cell body / cell body /  microtubule / Potential therapeutics for SARS / microtubule / Potential therapeutics for SARS /  cytoskeleton / cytoskeleton /  membrane raft / protein domain specific binding / membrane raft / protein domain specific binding /  cell division / cell division /  GTPase activity / GTPase activity /  ubiquitin protein ligase binding / Neutrophil degranulation / protein-containing complex binding / GTP binding / structural molecule activity / protein-containing complex / extracellular exosome / extracellular region / ubiquitin protein ligase binding / Neutrophil degranulation / protein-containing complex binding / GTP binding / structural molecule activity / protein-containing complex / extracellular exosome / extracellular region /  ATP binding / ATP binding /  metal ion binding / metal ion binding /  nucleus / nucleus /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) / Homo sapiens (human) /   Mus musculus (house mouse) Mus musculus (house mouse) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.7 Å cryo EM / Resolution: 3.7 Å | |||||||||
 Authors Authors | Mahalingan KK / Grotjahn D / Li Y / Lander GC / Zehr EA / Roll-Mecak A | |||||||||
| Funding support |  United States, 1 items United States, 1 items
| |||||||||
 Citation Citation |  Journal: Nat Chem Biol / Year: 2024 Journal: Nat Chem Biol / Year: 2024Title: Structural basis for α-tubulin-specific and modification state-dependent glutamylation. Authors: Kishore K Mahalingan / Danielle A Grotjahn / Yan Li / Gabriel C Lander / Elena A Zehr / Antonina Roll-Mecak /  Abstract: Microtubules have spatiotemporally complex posttranslational modification patterns. Tubulin tyrosine ligase-like (TTLL) enzymes introduce the most prevalent modifications on α-tubulin and β-tubulin. ...Microtubules have spatiotemporally complex posttranslational modification patterns. Tubulin tyrosine ligase-like (TTLL) enzymes introduce the most prevalent modifications on α-tubulin and β-tubulin. How TTLLs specialize for specific substrate recognition and ultimately modification-pattern generation is largely unknown. TTLL6, a glutamylase implicated in ciliopathies, preferentially modifies tubulin α-tails in microtubules. Cryo-electron microscopy, kinetic analysis and single-molecule biochemistry reveal an unprecedented quadrivalent recognition that ensures simultaneous readout of microtubule geometry and posttranslational modification status. By binding to a β-tubulin subunit, TTLL6 modifies the α-tail of the longitudinally adjacent tubulin dimer. Spanning two tubulin dimers along and across protofilaments (PFs) ensures fidelity of recognition of both the α-tail and the microtubule. Moreover, TTLL6 reads out and is stimulated by glutamylation of the β-tail of the laterally adjacent tubulin dimer, mediating crosstalk between α-tail and β-tail. This positive feedback loop can generate localized microtubule glutamylation patterns. Our work uncovers general principles that generate tubulin chemical and topographic complexity. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_41090.map.gz emd_41090.map.gz | 7.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-41090-v30.xml emd-41090-v30.xml emd-41090.xml emd-41090.xml | 23.2 KB 23.2 KB | Display Display |  EMDB header EMDB header |
| FSC (resolution estimation) |  emd_41090_fsc.xml emd_41090_fsc.xml | 20.1 KB | Display |  FSC data file FSC data file |
| Images |  emd_41090.png emd_41090.png | 151.2 KB | ||
| Filedesc metadata |  emd-41090.cif.gz emd-41090.cif.gz | 7.2 KB | ||
| Others |  emd_41090_half_map_1.map.gz emd_41090_half_map_1.map.gz emd_41090_half_map_2.map.gz emd_41090_half_map_2.map.gz | 562.1 MB 558.9 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-41090 http://ftp.pdbj.org/pub/emdb/structures/EMD-41090 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41090 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-41090 | HTTPS FTP |
-Related structure data
| Related structure data |  8u3zMC  8t42C C: citing same article ( M: atomic model generated by this map |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_41090.map.gz / Format: CCP4 / Size: 699 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_41090.map.gz / Format: CCP4 / Size: 699 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Composite map of TTLL6 bound to unmodified human microtubules. | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1.15 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Second half-map of TTLL6 bound to unmodified human microtubules.
| File | emd_41090_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Second half-map of TTLL6 bound to unmodified human microtubules. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: First half-map of TTLL6 bound to unmodified human microtubules.
| File | emd_41090_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | First half-map of TTLL6 bound to unmodified human microtubules. | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : TTLL6 bound to unmodified human microtubule
| Entire | Name: TTLL6 bound to unmodified human microtubule |
|---|---|
| Components |
|
-Supramolecule #1: TTLL6 bound to unmodified human microtubule
| Supramolecule | Name: TTLL6 bound to unmodified human microtubule / type: complex / ID: 1 / Parent: 0 / Macromolecule list: all Details: Microtubules were decorated with TTLL6 on electron microscopy grids |
|---|---|
| Molecular weight | Theoretical: 100 KDa |
-Supramolecule #2: alpha1B and betaI/betaIVb microtubule
| Supramolecule | Name: alpha1B and betaI/betaIVb microtubule / type: complex / ID: 2 / Parent: 1 / Macromolecule list: #1-#2 |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) / Organ: kidney / Location in cell: cytoplasm Homo sapiens (human) / Organ: kidney / Location in cell: cytoplasm |
-Supramolecule #3: Tubulin polyglutamylase TTLL6 in complex with unmodified human mi...
| Supramolecule | Name: Tubulin polyglutamylase TTLL6 in complex with unmodified human microtubule type: complex / ID: 3 / Parent: 1 / Macromolecule list: #3 |
|---|---|
| Source (natural) | Organism:   Mus musculus (house mouse) / Location in cell: cytoplasm Mus musculus (house mouse) / Location in cell: cytoplasm |
-Macromolecule #1: Tubulin alpha-1B chain
| Macromolecule | Name: Tubulin alpha-1B chain / type: other / ID: 1 / Classification: other |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) / Organ: kidney / Tissue: kidney Homo sapiens (human) / Organ: kidney / Tissue: kidney |
| Sequence | String: MRECISIHVG QAGVQIGNAC WELYCLEHGI QPDGQMPSDK TIGGGDDSFN TFFSETGAGK HVPRAVFVD LEPTVIDEVR TGTYRQLFHP EQLITGKEDA ANNYARGHYT IGKEIIDLVL D RIRKLADQ CTGLQGFLVF HSFGGGTGSG FTSLLMERLS VDYGKKSKLE ...String: MRECISIHVG QAGVQIGNAC WELYCLEHGI QPDGQMPSDK TIGGGDDSFN TFFSETGAGK HVPRAVFVD LEPTVIDEVR TGTYRQLFHP EQLITGKEDA ANNYARGHYT IGKEIIDLVL D RIRKLADQ CTGLQGFLVF HSFGGGTGSG FTSLLMERLS VDYGKKSKLE FSIYPAPQVS TA VVEPYNS ILTTHTTLEH SDCAFMVDNE AIYDICRRNL DIERPTYTNL NRLISQIVSS ITA SLRFDG ALNVDLTEFQ TNLVPYPRIH FPLATYAPVI SAEKAYHEQL SVAEITNACF EPAN QMVKC DPRHGKYMAC CLLYRGDVVP KDVNAAIATI KTKRSIQFVD WCPTGFKVGI NYQPP TVVP GGDLAKVQRA VCMLSNTTAI AEAWARLDHK FDLMYAKRAF VHWYVGEGME EGEFSE ARE DMAALEKDYE EVGVDSVEGE GEEEGEEY UniProtKB: Tubulin alpha-1B chain |
-Macromolecule #2: Tubulin beta chain
| Macromolecule | Name: Tubulin beta chain / type: other / ID: 2 / Classification: other |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) / Organ: kidney / Tissue: kidney Homo sapiens (human) / Organ: kidney / Tissue: kidney |
| Sequence | String: MREIVHIQAG QCGNQIGAKF WEVISDEHGI DPTGTYHGDS DLQLDRISVY YNEATGGKYV PRAILVDLE PGTMDSVRSG PFGQIFRPDN FVFGQSGAGN NWAKGHYTEG AELVDSVLDV V RKEAESCD CLQGFQLTHS LGGGTGSGMG TLLISKIREE YPDRIMNTFS ...String: MREIVHIQAG QCGNQIGAKF WEVISDEHGI DPTGTYHGDS DLQLDRISVY YNEATGGKYV PRAILVDLE PGTMDSVRSG PFGQIFRPDN FVFGQSGAGN NWAKGHYTEG AELVDSVLDV V RKEAESCD CLQGFQLTHS LGGGTGSGMG TLLISKIREE YPDRIMNTFS VVPSPKVSDT VV EPYNATL SVHQLVENTD ETYCIDNEAL YDICFRTLKL TTPTYGDLNH LVSATMSGVT TCL RFPGQL NADLRKLAVN MVPFPRLHFF MPGFAPLTSR GSQQYRALTV PELTQQVFDA KNMM AACDP RHGRYLTVAA VFRGRMSMKE VDEQMLNVQN KNSSYFVEWI PNNVKTAVCD IPPRG LKMA VTFIGNSTAI QELFKRISEQ FTAMFRRKAF LHWYTGEGMD EMEFTEAESN MNDLVS EYQ QYQDATAEEE EDFGEEAEEE A UniProtKB: Tubulin beta chain |
-Macromolecule #3: Tubulin polyglutamylase TTLL6
| Macromolecule | Name: Tubulin polyglutamylase TTLL6 / type: other / ID: 3 / Classification: other |
|---|---|
| Source (natural) | Organism:   Mus musculus (house mouse) Mus musculus (house mouse) |
| Sequence | String: GKKKRKKKRL VINLSNCRYD SVRRAAQQYG LREAGDNDDW TLYWTDYSVS LERVMEMKSY QKINHFPGMS EICRKDLLAR NMSRMLKLFP KDFHFFPRTW CLPADWGDLQ TYSRTRKNKT YICKPDSGCQ GRGIFITRSV KEIKPGEDMI CQLYISKPFI IDGFKFDLRV ...String: GKKKRKKKRL VINLSNCRYD SVRRAAQQYG LREAGDNDDW TLYWTDYSVS LERVMEMKSY QKINHFPGMS EICRKDLLAR NMSRMLKLFP KDFHFFPRTW CLPADWGDLQ TYSRTRKNKT YICKPDSGCQ GRGIFITRSV KEIKPGEDMI CQLYISKPFI IDGFKFDLRV YVLVTSCDPL RVFVYNEGLA RFATTSYSHP NLDNLDEICM HLTNYSINKH SSNFVQDAFS GSKRKLSTFN SYMKTHGYDV EQIWRGIEDV IIKTLISAHP VIKHNYHTCF PSHTLNSACF EILGFDILLD RKLKPWLLEV NHSPSFSTDS KLDKEVKDSL LYDALVLINL GNCDKKKVLE EERQRGRFLQ QCPNREIRLE EVKGFQAMRL QKTEEYEKKN CGGFRLIYPG LNLEKYDKFF QDNSSLFQNT VASRARELYA RQLIQELRQK QEKKVFLKKA RKA UniProtKB: Tubulin polyglutamylase TTLL6 |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | filament |
- Sample preparation
Sample preparation
| Concentration | 0.1 mg/mL | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Buffer | pH: 7 Component:
| ||||||||||||||||||
| Grid | Model: Quantifoil R1.2/1.3 / Material: GOLD / Mesh: 300 / Support film - Material: GOLD / Support film - topology: HOLEY / Support film - Film thickness: 100 | ||||||||||||||||||
| Vitrification | Cryogen name: ETHANE / Chamber humidity: 100 % / Chamber temperature: 303 K / Instrument: FEI VITROBOT MARK IV Details: The sample was blotted with Whatman #5 blotting paper on both sides of the grid for 3 s with a blot offset of -1 using a Vitrobot Mark IV (Thermo Fisher Scientific) with a chamber set to 30C ...Details: The sample was blotted with Whatman #5 blotting paper on both sides of the grid for 3 s with a blot offset of -1 using a Vitrobot Mark IV (Thermo Fisher Scientific) with a chamber set to 30C at 100% humidity and subsequently plunged into liquid ethane.. | ||||||||||||||||||
| Details | TTLL6 decoration of microtubule was heterogenous |
- Electron microscopy
Electron microscopy
| Microscope | FEI TALOS ARCTICA |
|---|---|
| Electron beam | Acceleration voltage: 200 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: FLOOD BEAM / Imaging mode: BRIGHT FIELD Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 36000 Bright-field microscopy / Cs: 2.7 mm / Nominal defocus max: 2.0 µm / Nominal defocus min: 1.0 µm / Nominal magnification: 36000 |
| Image recording | Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Detector mode: COUNTING / Digitization - Frames/image: 1-39 / Number real images: 2771 / Average exposure time: 9.75 sec. / Average electron dose: 42.0 e/Å2 |
| Experimental equipment |  Model: Talos Arctica / Image courtesy: FEI Company |
- Image processing
Image processing
-Atomic model buiding 1
| Initial model |
| ||||||
|---|---|---|---|---|---|---|---|
| Refinement | Space: REAL / Protocol: FLEXIBLE FIT / Target criteria: Cross-correlation | ||||||
| Output model |  PDB-8u3z: |
 Movie
Movie Controller
Controller


































 Z
Z Y
Y X
X




















