[English] 日本語
 Yorodumi
Yorodumi- PDB-8qg0: Archaeoglobus fulgidus AfAgo complex with AfAgo-N protein (fAfAgo... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8qg0 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Archaeoglobus fulgidus AfAgo complex with AfAgo-N protein (fAfAgo) bound with 17 nt RNA guide and 17 nt DNA target | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords |  DNA BINDING PROTEIN / DNA BINDING PROTEIN /  ARGONAUTE / ARGONAUTE /  PIWI DOMAIN / PIWI DOMAIN /  PROTEIN-DNA COMPLEX PROTEIN-DNA COMPLEX | |||||||||
| Function / homology |  Function and homology information Function and homology information | |||||||||
| Biological species |    Archaeoglobus fulgidus (archaea) Archaeoglobus fulgidus (archaea)  Escherichia coli (E. coli) Escherichia coli (E. coli) | |||||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.43 Å cryo EM / Resolution: 3.43 Å | |||||||||
 Authors Authors | Manakova, E.N. / Zaremba, M. / Pocevicuite, R. / Golovinas, E. / Zagorskaite, E. / Silanskas, A. | |||||||||
| Funding support | Lithuania, 2items
| |||||||||
 Citation Citation |  Journal: Nucleic Acids Res / Year: 2024 Journal: Nucleic Acids Res / Year: 2024Title: The missing part: the Archaeoglobus fulgidus Argonaute forms a functional heterodimer with an N-L1-L2 domain protein. Authors: Elena Manakova / Edvardas Golovinas / Reda Pocevičiūtė / Giedrius Sasnauskas / Arunas Silanskas / Danielis Rutkauskas / Marija Jankunec / Evelina Zagorskaitė / Edvinas Jurgelaitis / ...Authors: Elena Manakova / Edvardas Golovinas / Reda Pocevičiūtė / Giedrius Sasnauskas / Arunas Silanskas / Danielis Rutkauskas / Marija Jankunec / Evelina Zagorskaitė / Edvinas Jurgelaitis / Algirdas Grybauskas / Česlovas Venclovas / Mindaugas Zaremba Abstract: Argonaute (Ago) proteins are present in all three domains of life (bacteria, archaea and eukaryotes). They use small (15-30 nucleotides) oligonucleotide guides to bind complementary nucleic acid ...Argonaute (Ago) proteins are present in all three domains of life (bacteria, archaea and eukaryotes). They use small (15-30 nucleotides) oligonucleotide guides to bind complementary nucleic acid targets and are responsible for gene expression regulation, mobile genome element silencing, and defence against viruses or plasmids. According to their domain organization, Agos are divided into long and short Agos. Long Agos found in prokaryotes (long-A and long-B pAgos) and eukaryotes (eAgos) comprise four major functional domains (N, PAZ, MID and PIWI) and two structural linker domains L1 and L2. The majority (∼60%) of pAgos are short pAgos, containing only the MID and inactive PIWI domains. Here we focus on the prokaryotic Argonaute AfAgo from Archaeoglobus fulgidus DSM4304. Although phylogenetically classified as a long-B pAgo, AfAgo contains only MID and catalytically inactive PIWI domains, akin to short pAgos. We show that AfAgo forms a heterodimeric complex with a protein encoded upstream in the same operon, which is a structural equivalent of the N-L1-L2 domains of long pAgos. This complex, structurally equivalent to a long PAZ-less pAgo, outperforms standalone AfAgo in guide RNA-mediated target DNA binding. Our findings provide a missing piece to one of the first and the most studied pAgos. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8qg0.cif.gz 8qg0.cif.gz | 151.4 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8qg0.ent.gz pdb8qg0.ent.gz | 111 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8qg0.json.gz 8qg0.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/qg/8qg0 https://data.pdbj.org/pub/pdb/validation_reports/qg/8qg0 ftp://data.pdbj.org/pub/pdb/validation_reports/qg/8qg0 ftp://data.pdbj.org/pub/pdb/validation_reports/qg/8qg0 | HTTPS FTP |
|---|
-Related structure data
| Related structure data | 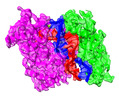 18386MC  8ok9C  8oldC  8oljC  8pvvC C: citing same article ( M: map data used to model this data |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
| Other databases |
|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein |  Mass: 49302.434 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)    Archaeoglobus fulgidus (archaea) / Strain: DSM4304 / Gene: XD48_2091 / Plasmid: pBAD / Production host: Archaeoglobus fulgidus (archaea) / Strain: DSM4304 / Gene: XD48_2091 / Plasmid: pBAD / Production host:   Escherichia coli BL21(DE3) (bacteria) / References: UniProt: A0A101DYI0 Escherichia coli BL21(DE3) (bacteria) / References: UniProt: A0A101DYI0 |
|---|---|
| #2: Protein | Mass: 31356.576 Da / Num. of mol.: 1 / Mutation: N-terminal His-tag Source method: isolated from a genetically manipulated source Source: (gene. exp.)    Archaeoglobus fulgidus (archaea) / Strain: DSM8774 / Gene: AFULGI_00014290 / Plasmid: pBAD / Production host: Archaeoglobus fulgidus (archaea) / Strain: DSM8774 / Gene: AFULGI_00014290 / Plasmid: pBAD / Production host:   Escherichia coli BL21(DE3) (bacteria) / References: UniProt: A0A075WKW4 Escherichia coli BL21(DE3) (bacteria) / References: UniProt: A0A075WKW4 |
| #3: DNA chain | Mass: 5202.384 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.)   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| #4: RNA chain | Mass: 5427.286 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.)   Escherichia coli (E. coli) Escherichia coli (E. coli) |
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component |
| ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 0.1 MDa / Experimental value: YES | ||||||||||||||||||||||||||||||||||||
| Source (natural) |
| ||||||||||||||||||||||||||||||||||||
| Source (recombinant) |
| ||||||||||||||||||||||||||||||||||||
| Buffer solution | pH: 8.5 | ||||||||||||||||||||||||||||||||||||
| Buffer component |
| ||||||||||||||||||||||||||||||||||||
| Specimen | Conc.: 1 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YESDetails: fAfAgo complex with 17/17 guide-target heteroduplexes was mixed and applied on grid | ||||||||||||||||||||||||||||||||||||
| Specimen support | Grid material: COPPER / Grid mesh size: 300 divisions/in. / Grid type: Quantifoil R1.2/1.3 | ||||||||||||||||||||||||||||||||||||
Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 95 % / Chamber temperature: 277 K |
- Electron microscopy imaging
Electron microscopy imaging
| Microscopy | Model: TFS GLACIOS |
|---|---|
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 200 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal magnification: 92000 X / Nominal defocus max: 2000 nm / Nominal defocus min: 1000 nm / Cs Bright-field microscopy / Nominal magnification: 92000 X / Nominal defocus max: 2000 nm / Nominal defocus min: 1000 nm / Cs : 2.7 mm / C2 aperture diameter: 100 µm / Alignment procedure: BASIC : 2.7 mm / C2 aperture diameter: 100 µm / Alignment procedure: BASIC |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: OTHER |
| Image recording | Average exposure time: 46.33 sec. / Electron dose: 31 e/Å2 / Detector mode: COUNTING / Film or detector model: FEI FALCON III (4k x 4k) / Num. of grids imaged: 1 / Num. of real images: 2152 |
| Image scans | Width: 4000 / Height: 4000 |
- Processing
Processing
| EM software |
| ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
CTF correction | Type: NONE | ||||||||||||||||||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 1480423 / Details: Blob particle picking | ||||||||||||||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry : C1 (asymmetric) : C1 (asymmetric) | ||||||||||||||||||||||||||||||||||||||||||||||||
3D reconstruction | Resolution: 3.43 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 364005 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | B value: 48 / Protocol: FLEXIBLE FIT / Space: REAL | ||||||||||||||||||||||||||||||||||||||||||||||||
| Atomic model building | 3D fitting-ID: 1 / Accession code: 8ok9 / Details: fAfAgo complex was used as initial model / Initial refinement model-ID: 1 / PDB-ID: 8ok9 / Source name: PDB / Type: experimental model
| ||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller



 PDBj
PDBj































































