[English] 日本語
 Yorodumi
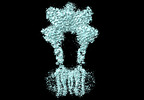
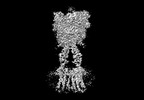
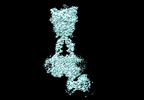
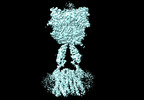
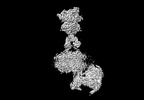
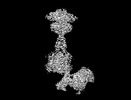
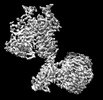
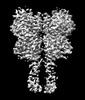
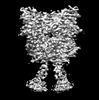
Yorodumi- PDB-8jcw: Cryo-EM structure of mGlu2-mGlu3 heterodimer in presence of LY341... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8jcw | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Cryo-EM structure of mGlu2-mGlu3 heterodimer in presence of LY341495 and NAM563 (dimerization mode I) | |||||||||
 Components Components |
| |||||||||
 Keywords Keywords |  MEMBRANE PROTEIN / Complex structure / mGlu2-3 heterodimer MEMBRANE PROTEIN / Complex structure / mGlu2-3 heterodimer | |||||||||
| Function / homology |  Function and homology information Function and homology informationpositive regulation of response to stimulus / regulation of cellular component organization / regulation of response to drug / group II metabotropic glutamate receptor activity / response to chemical / regulation of cellular response to stress /  macrolide binding / macrolide binding /  TORC1 complex / TORC1 complex /  activin receptor binding / cytoplasmic side of membrane ...positive regulation of response to stimulus / regulation of cellular component organization / regulation of response to drug / group II metabotropic glutamate receptor activity / response to chemical / regulation of cellular response to stress / activin receptor binding / cytoplasmic side of membrane ...positive regulation of response to stimulus / regulation of cellular component organization / regulation of response to drug / group II metabotropic glutamate receptor activity / response to chemical / regulation of cellular response to stress /  macrolide binding / macrolide binding /  TORC1 complex / TORC1 complex /  activin receptor binding / cytoplasmic side of membrane / behavioral response to nicotine / G protein-coupled glutamate receptor signaling pathway / intracellular glutamate homeostasis / activin receptor binding / cytoplasmic side of membrane / behavioral response to nicotine / G protein-coupled glutamate receptor signaling pathway / intracellular glutamate homeostasis /  transforming growth factor beta receptor binding / TGFBR1 LBD Mutants in Cancer / signaling receptor inhibitor activity / astrocyte projection / type I transforming growth factor beta receptor binding / negative regulation of adenylate cyclase activity / Class C/3 (Metabotropic glutamate/pheromone receptors) / negative regulation of activin receptor signaling pathway / heart trabecula formation / glutamate secretion / transforming growth factor beta receptor binding / TGFBR1 LBD Mutants in Cancer / signaling receptor inhibitor activity / astrocyte projection / type I transforming growth factor beta receptor binding / negative regulation of adenylate cyclase activity / Class C/3 (Metabotropic glutamate/pheromone receptors) / negative regulation of activin receptor signaling pathway / heart trabecula formation / glutamate secretion /  terminal cisterna / terminal cisterna /  ryanodine receptor complex / ryanodine receptor complex /  glutamate receptor activity / regulation of glutamate secretion / glutamate receptor activity / regulation of glutamate secretion /  I-SMAD binding / regulation of amyloid precursor protein catabolic process / long-term synaptic depression / protein maturation by protein folding / 'de novo' protein folding / ventricular cardiac muscle tissue morphogenesis / negative regulation of phosphoprotein phosphatase activity / I-SMAD binding / regulation of amyloid precursor protein catabolic process / long-term synaptic depression / protein maturation by protein folding / 'de novo' protein folding / ventricular cardiac muscle tissue morphogenesis / negative regulation of phosphoprotein phosphatase activity /  FK506 binding / postsynaptic modulation of chemical synaptic transmission / regulation of dopamine secretion / mTORC1-mediated signalling / TGF-beta receptor signaling activates SMADs / calcium channel regulator activity / Calcineurin activates NFAT / FK506 binding / postsynaptic modulation of chemical synaptic transmission / regulation of dopamine secretion / mTORC1-mediated signalling / TGF-beta receptor signaling activates SMADs / calcium channel regulator activity / Calcineurin activates NFAT /  regulation of immune response / protein peptidyl-prolyl isomerization / supramolecular fiber organization / heart morphogenesis / regulation of ryanodine-sensitive calcium-release channel activity / regulation of immune response / protein peptidyl-prolyl isomerization / supramolecular fiber organization / heart morphogenesis / regulation of ryanodine-sensitive calcium-release channel activity /  regulation of synaptic transmission, glutamatergic / sarcoplasmic reticulum membrane / presynaptic modulation of chemical synaptic transmission / positive regulation of protein metabolic process / regulation of synaptic transmission, glutamatergic / sarcoplasmic reticulum membrane / presynaptic modulation of chemical synaptic transmission / positive regulation of protein metabolic process /  T cell activation / negative regulation of autophagy / response to cocaine / TGF-beta receptor signaling in EMT (epithelial to mesenchymal transition) / T cell activation / negative regulation of autophagy / response to cocaine / TGF-beta receptor signaling in EMT (epithelial to mesenchymal transition) /  sarcoplasmic reticulum / sarcoplasmic reticulum /  peptidylprolyl isomerase / peptidylprolyl isomerase /  peptidyl-prolyl cis-trans isomerase activity / G protein-coupled receptor activity / calcium ion transmembrane transport / negative regulation of transforming growth factor beta receptor signaling pathway / Z disc / SARS-CoV-1 activates/modulates innate immune responses / peptidyl-prolyl cis-trans isomerase activity / G protein-coupled receptor activity / calcium ion transmembrane transport / negative regulation of transforming growth factor beta receptor signaling pathway / Z disc / SARS-CoV-1 activates/modulates innate immune responses /  regulation of protein localization / regulation of protein localization /  protein folding / protein folding /  presynaptic membrane / presynaptic membrane /  gene expression / positive regulation of protein binding / G alpha (i) signalling events / protein refolding / chemical synaptic transmission / gene expression / positive regulation of protein binding / G alpha (i) signalling events / protein refolding / chemical synaptic transmission /  scaffold protein binding / scaffold protein binding /  postsynaptic membrane / positive regulation of canonical NF-kappaB signal transduction / Potential therapeutics for SARS / transmembrane transporter binding / amyloid fibril formation / postsynaptic membrane / positive regulation of canonical NF-kappaB signal transduction / Potential therapeutics for SARS / transmembrane transporter binding / amyloid fibril formation /  dendritic spine / dendritic spine /  postsynaptic density / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction / postsynaptic density / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction /  non-specific serine/threonine protein kinase / non-specific serine/threonine protein kinase /  axon / axon /  phosphorylation / protein serine/threonine kinase activity / phosphorylation / protein serine/threonine kinase activity /  dendrite / glutamatergic synapse / protein-containing complex binding / dendrite / glutamatergic synapse / protein-containing complex binding /  ATP binding / ATP binding /  membrane / membrane /  plasma membrane / plasma membrane /  cytosol / cytosol /  cytoplasm cytoplasmSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | |||||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3 Å cryo EM / Resolution: 3 Å | |||||||||
 Authors Authors | Wang, X. / Wang, M. / Xu, T. / Feng, Y. / Han, S. / Lin, S. / Zhao, Q. / Wu, B. | |||||||||
| Funding support |  China, 2items China, 2items
| |||||||||
 Citation Citation |  Journal: Cell Res / Year: 2023 Journal: Cell Res / Year: 2023Title: Structural insights into dimerization and activation of the mGlu2-mGlu3 and mGlu2-mGlu4 heterodimers. Authors: Xinwei Wang / Mu Wang / Tuo Xu / Ye Feng / Qiang Shao / Shuo Han / Xiaojing Chu / Yechun Xu / Shuling Lin / Qiang Zhao / Beili Wu /  Abstract: Heterodimerization of the metabotropic glutamate receptors (mGlus) has shown importance in the functional modulation of the receptors and offers potential drug targets for treating central nervous ...Heterodimerization of the metabotropic glutamate receptors (mGlus) has shown importance in the functional modulation of the receptors and offers potential drug targets for treating central nervous system diseases. However, due to a lack of molecular details of the mGlu heterodimers, understanding of the mechanisms underlying mGlu heterodimerization and activation is limited. Here we report twelve cryo-electron microscopy (cryo-EM) structures of the mGlu2-mGlu3 and mGlu2-mGlu4 heterodimers in different conformational states, including inactive, intermediate inactive, intermediate active and fully active conformations. These structures provide a full picture of conformational rearrangement of mGlu2-mGlu3 upon activation. The Venus flytrap domains undergo a sequential conformational change, while the transmembrane domains exhibit a substantial rearrangement from an inactive, symmetric dimer with diverse dimerization patterns to an active, asymmetric dimer in a conserved dimerization mode. Combined with functional data, these structures reveal that stability of the inactive conformations of the subunits and the subunit-G protein interaction pattern are determinants of asymmetric signal transduction of the heterodimers. Furthermore, a novel binding site for two mGlu4 positive allosteric modulators was observed in the asymmetric dimer interfaces of the mGlu2-mGlu4 heterodimer and mGlu4 homodimer, and may serve as a drug recognition site. These findings greatly extend our knowledge about signal transduction of the mGlus. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8jcw.cif.gz 8jcw.cif.gz | 280 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8jcw.ent.gz pdb8jcw.ent.gz | 204.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8jcw.json.gz 8jcw.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/jc/8jcw https://data.pdbj.org/pub/pdb/validation_reports/jc/8jcw ftp://data.pdbj.org/pub/pdb/validation_reports/jc/8jcw ftp://data.pdbj.org/pub/pdb/validation_reports/jc/8jcw | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  36167MC  8jcuC  8jcvC  8jcxC  8jcyC  8jczC  8jd0C  8jd1C  8jd2C  8jd3C  8jd4C  8jd5C  8jd6C M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: Protein | Mass: 109398.914 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: GRM2, GPRC1B, MGLUR2, FKBP1A, FKBP1, FKBP12 Homo sapiens (human) / Gene: GRM2, GPRC1B, MGLUR2, FKBP1A, FKBP1, FKBP12Production host:  References: UniProt: Q14416, UniProt: P62942,  peptidylprolyl isomerase peptidylprolyl isomerase | ||||||
|---|---|---|---|---|---|---|---|
| #2: Protein | Mass: 112712.961 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: GRM3, GPRC1C, MGLUR3, MTOR Homo sapiens (human) / Gene: GRM3, GPRC1C, MGLUR3, MTORProduction host:  References: UniProt: Q14832, UniProt: A0A8V8TRG9,  non-specific serine/threonine protein kinase non-specific serine/threonine protein kinase | ||||||
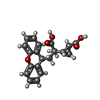
| #3: Sugar |  N-Acetylglucosamine N-Acetylglucosamine#4: Chemical |  LY-341495 LY-341495#5: Chemical |  Cholesterol CholesterolHas ligand of interest | Y | |
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: mGlu2-3 heterodimer in presence of LY341495 and NAM563 Type: COMPLEX / Entity ID: #1-#2 / Source: MULTIPLE SOURCES |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:  |
| Buffer solution | pH: 7.5 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES |
Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: SPOT SCAN FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: SPOT SCAN |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal defocus max: 1500 nm / Nominal defocus min: 800 nm Bright-field microscopy / Nominal defocus max: 1500 nm / Nominal defocus min: 800 nm |
| Image recording | Electron dose: 70 e/Å2 / Film or detector model: GATAN K3 BIOQUANTUM (6k x 4k) |
- Processing
Processing
CTF correction | Type: NONE | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
3D reconstruction | Resolution: 3 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 516615 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refinement | Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2 | ||||||||||||||||||||||||
| Displacement parameters | Biso mean: 65.76 Å2 | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller











 PDBj
PDBj