[English] 日本語
 Yorodumi
Yorodumi- PDB-8jbf: Senktide bound to active human neurokinin 3 receptor in complex w... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8jbf | ||||||
|---|---|---|---|---|---|---|---|
| Title | Senktide bound to active human neurokinin 3 receptor in complex with Gq | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  MEMBRANE PROTEIN / MEMBRANE PROTEIN /  G protein coupled receptor / G protein coupled receptor /  Neurokinin / Neurokinin /  Cryo-EM / Peptide Agonists Cryo-EM / Peptide Agonists | ||||||
| Function / homology |  Function and homology information Function and homology information tachykinin receptor activity / positive regulation of flagellated sperm motility / Tachykinin receptors bind tachykinins / positive regulation of uterine smooth muscle contraction / tachykinin receptor signaling pathway / tachykinin receptor activity / positive regulation of flagellated sperm motility / Tachykinin receptors bind tachykinins / positive regulation of uterine smooth muscle contraction / tachykinin receptor signaling pathway /  regulation of feeding behavior / regulation of dopamine metabolic process / positive regulation of blood pressure / G-protein activation / Activation of the phototransduction cascade ... regulation of feeding behavior / regulation of dopamine metabolic process / positive regulation of blood pressure / G-protein activation / Activation of the phototransduction cascade ... tachykinin receptor activity / positive regulation of flagellated sperm motility / Tachykinin receptors bind tachykinins / positive regulation of uterine smooth muscle contraction / tachykinin receptor signaling pathway / tachykinin receptor activity / positive regulation of flagellated sperm motility / Tachykinin receptors bind tachykinins / positive regulation of uterine smooth muscle contraction / tachykinin receptor signaling pathway /  regulation of feeding behavior / regulation of dopamine metabolic process / positive regulation of blood pressure / G-protein activation / Activation of the phototransduction cascade / : / Glucagon-type ligand receptors / Thromboxane signalling through TP receptor / Sensory perception of sweet, bitter, and umami (glutamate) taste / G beta:gamma signalling through PI3Kgamma / G beta:gamma signalling through CDC42 / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / Ca2+ pathway / Activation of G protein gated Potassium channels / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G alpha (z) signalling events / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / Adrenaline,noradrenaline inhibits insulin secretion / ADP signalling through P2Y purinoceptor 12 / G alpha (q) signalling events / G alpha (i) signalling events / Thrombin signalling through proteinase activated receptors (PARs) / Activation of G protein gated Potassium channels / G-protein activation / G beta:gamma signalling through PI3Kgamma / Prostacyclin signalling through prostacyclin receptor / G beta:gamma signalling through PLC beta / ADP signalling through P2Y purinoceptor 1 / Thromboxane signalling through TP receptor / Presynaptic function of Kainate receptors / G beta:gamma signalling through CDC42 / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Glucagon-type ligand receptors / regulation of feeding behavior / regulation of dopamine metabolic process / positive regulation of blood pressure / G-protein activation / Activation of the phototransduction cascade / : / Glucagon-type ligand receptors / Thromboxane signalling through TP receptor / Sensory perception of sweet, bitter, and umami (glutamate) taste / G beta:gamma signalling through PI3Kgamma / G beta:gamma signalling through CDC42 / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / Ca2+ pathway / Activation of G protein gated Potassium channels / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G alpha (z) signalling events / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / Adrenaline,noradrenaline inhibits insulin secretion / ADP signalling through P2Y purinoceptor 12 / G alpha (q) signalling events / G alpha (i) signalling events / Thrombin signalling through proteinase activated receptors (PARs) / Activation of G protein gated Potassium channels / G-protein activation / G beta:gamma signalling through PI3Kgamma / Prostacyclin signalling through prostacyclin receptor / G beta:gamma signalling through PLC beta / ADP signalling through P2Y purinoceptor 1 / Thromboxane signalling through TP receptor / Presynaptic function of Kainate receptors / G beta:gamma signalling through CDC42 / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Glucagon-type ligand receptors /  alkylglycerophosphoethanolamine phosphodiesterase activity / Adrenaline,noradrenaline inhibits insulin secretion / G alpha (12/13) signalling events / G beta:gamma signalling through BTK / ADP signalling through P2Y purinoceptor 12 / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / Thrombin signalling through proteinase activated receptors (PARs) / Ca2+ pathway / G alpha (z) signalling events / Extra-nuclear estrogen signaling / G alpha (s) signalling events / G alpha (q) signalling events / photoreceptor outer segment membrane / G alpha (i) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / neuronal cell body membrane / alkylglycerophosphoethanolamine phosphodiesterase activity / Adrenaline,noradrenaline inhibits insulin secretion / G alpha (12/13) signalling events / G beta:gamma signalling through BTK / ADP signalling through P2Y purinoceptor 12 / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / Thrombin signalling through proteinase activated receptors (PARs) / Ca2+ pathway / G alpha (z) signalling events / Extra-nuclear estrogen signaling / G alpha (s) signalling events / G alpha (q) signalling events / photoreceptor outer segment membrane / G alpha (i) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / neuronal cell body membrane /  spectrin binding / positive regulation of heart rate / Vasopressin regulates renal water homeostasis via Aquaporins / photoreceptor outer segment / cardiac muscle cell apoptotic process / sperm midpiece / dendrite membrane / photoreceptor inner segment / response to cocaine / cellular response to catecholamine stimulus / adenylate cyclase-activating dopamine receptor signaling pathway / cellular response to prostaglandin E stimulus / sensory perception of taste / G-protein beta-subunit binding / spectrin binding / positive regulation of heart rate / Vasopressin regulates renal water homeostasis via Aquaporins / photoreceptor outer segment / cardiac muscle cell apoptotic process / sperm midpiece / dendrite membrane / photoreceptor inner segment / response to cocaine / cellular response to catecholamine stimulus / adenylate cyclase-activating dopamine receptor signaling pathway / cellular response to prostaglandin E stimulus / sensory perception of taste / G-protein beta-subunit binding /  heterotrimeric G-protein complex / signaling receptor complex adaptor activity / response to estradiol / retina development in camera-type eye / heterotrimeric G-protein complex / signaling receptor complex adaptor activity / response to estradiol / retina development in camera-type eye /  GTPase binding / GTPase binding /  cell body / phospholipase C-activating G protein-coupled receptor signaling pathway / positive regulation of cytosolic calcium ion concentration / cellular response to hypoxia / G alpha (q) signalling events / cell population proliferation / G protein-coupled receptor signaling pathway / cell body / phospholipase C-activating G protein-coupled receptor signaling pathway / positive regulation of cytosolic calcium ion concentration / cellular response to hypoxia / G alpha (q) signalling events / cell population proliferation / G protein-coupled receptor signaling pathway /  GTPase activity / GTPase activity /  dendrite / protein-containing complex binding / dendrite / protein-containing complex binding /  membrane / membrane /  plasma membrane / plasma membrane /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human)  Rattus norvegicus (Norway rat) Rattus norvegicus (Norway rat)  Mus musculoides (Temminck's mouse) Mus musculoides (Temminck's mouse)  Bos taurus (cattle) Bos taurus (cattle) | ||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3 Å cryo EM / Resolution: 3 Å | ||||||
 Authors Authors | Sun, W.J. / Yang, F. / Zhang, H.H. / Yuan, Q.N. / Yin, W.C. / Shi, P. / Eric, X. / Tian, C.L. | ||||||
| Funding support |  China, 1items China, 1items
| ||||||
 Citation Citation |  Journal: Cell Discov / Year: 2023 Journal: Cell Discov / Year: 2023Title: Structural insights into neurokinin 3 receptor activation by endogenous and analogue peptide agonists. Authors: Wenjing Sun / Fan Yang / Huanhuan Zhang / Qingning Yuan / Shenglong Ling / Yuanxia Wang / Pei Lv / Zelin Li / Yifan Luo / Dongsheng Liu / Wanchao Yin / Pan Shi / H Eric Xu / Changlin Tian /  Abstract: Neurokinin 3 receptor (NK3R) is a tachykinin receptor essential for the hypothalamic-pituitary-gonadal axis. The endogenous peptide agonist neurokinin B (NKB) preferentially activates NK3R, while ...Neurokinin 3 receptor (NK3R) is a tachykinin receptor essential for the hypothalamic-pituitary-gonadal axis. The endogenous peptide agonist neurokinin B (NKB) preferentially activates NK3R, while substance P (SP) binds preferentially to NK1R. In addition, the SP analogue senktide more potently activates NK3R than NKB and SP. However, the mechanisms of preferential binding of peptide and NK3R activation remain elusive. Herein, we determined the cryogenic electron microscopy (cryo-EM) structures of the NK3R-G complex bound to NKB, SP and senktide. The three NK3R-G/peptide complexes utilize a class of noncanonical receptor activation mechanisms. Combining the structural analysis and functional assay illustrated that the consensus C-termini of the three peptide agonists share a conserved binding mode to NK3R, while the divergent N-termini of the peptides confer the preferential binding of the agonist to NK3R. In addition, the specific interactions between the N-terminus of senktide and the N-terminus and extracellular loops (ECL2 and ECL3) of NK3R lead to the improved activation displayed by senktide compared to SP and NKB. These findings pave the way to understand tachykinin receptor subtype selectivity and provide ideas to rationally develop drugs targeting NK3R. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8jbf.cif.gz 8jbf.cif.gz | 218.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8jbf.ent.gz pdb8jbf.ent.gz | 163.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8jbf.json.gz 8jbf.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/jb/8jbf https://data.pdbj.org/pub/pdb/validation_reports/jb/8jbf ftp://data.pdbj.org/pub/pdb/validation_reports/jb/8jbf ftp://data.pdbj.org/pub/pdb/validation_reports/jb/8jbf | HTTPS FTP |
|---|
-Related structure data
| Related structure data | 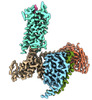 36144MC  8jbgC  8jbhC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-Guanine nucleotide-binding protein ... , 3 types, 3 molecules DGC
| #2: Protein | Mass: 41042.828 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Rattus norvegicus (Norway rat) / Gene: Gnb1 / Production host: Rattus norvegicus (Norway rat) / Gene: Gnb1 / Production host:   Spodoptera frugiperda (fall armyworm) / References: UniProt: P54311 Spodoptera frugiperda (fall armyworm) / References: UniProt: P54311 |
|---|---|
| #4: Protein | Mass: 7861.143 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Bos taurus (cattle) / Gene: GNG2 / Production host: Bos taurus (cattle) / Gene: GNG2 / Production host:   Spodoptera frugiperda (fall armyworm) / References: UniProt: P63212 Spodoptera frugiperda (fall armyworm) / References: UniProt: P63212 |
| #6: Protein | Mass: 41694.289 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Production host: Homo sapiens (human) / Production host:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
-Protein / Antibody / Protein/peptide , 3 types, 3 molecules BEA
| #1: Protein | Mass: 44317.020 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Details: NK3R-pFastbac1 / Source: (gene. exp.)   Homo sapiens (human) / Gene: TACR3, NK3R, TAC3R / Production host: Homo sapiens (human) / Gene: TACR3, NK3R, TAC3R / Production host:   Spodoptera frugiperda (fall armyworm) / References: UniProt: P29371 Spodoptera frugiperda (fall armyworm) / References: UniProt: P29371 |
|---|---|
| #3: Antibody | Mass: 32708.473 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Mus musculoides (Temminck's mouse) / Production host: Mus musculoides (Temminck's mouse) / Production host:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| #5: Protein/peptide | Mass: 915.022 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Production host: Homo sapiens (human) / Production host:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: Senktide bound to active human neurokinin 3 receptor in complex with Gq heterotrimer Type: COMPLEX / Entity ID: all / Source: RECOMBINANT |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Source (recombinant) | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Buffer solution | pH: 7.5 |
| Specimen | Conc.: 1 mg/ml / Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES |
Vitrification | Cryogen name: ETHANE-PROPANE / Humidity: 100 % |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: SPOT SCAN FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: SPOT SCAN |
| Electron lens | Mode: DIFFRACTION / Nominal defocus max: 2200 nm / Nominal defocus min: 1200 nm / Cs / Nominal defocus max: 2200 nm / Nominal defocus min: 1200 nm / Cs : 2.7 mm : 2.7 mm |
| Image recording | Electron dose: 50 e/Å2 / Film or detector model: GATAN K3 (6k x 4k) |
- Processing
Processing
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
3D reconstruction | Resolution: 3 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 2457160 / Symmetry type: POINT | ||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller




 PDBj
PDBj














