[English] 日本語
 Yorodumi
Yorodumi- EMDB-36144: Senktide bound to active human neurokinin 3 receptor in complex w... -
+ Open data
Open data
- Basic information
Basic information
| Entry | 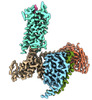 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Senktide bound to active human neurokinin 3 receptor in complex with Gq | |||||||||
 Map data Map data | Senktide bound to active human neurokinin 3 receptor in complex with Gq | |||||||||
 Sample Sample |
| |||||||||
 Keywords Keywords |  G protein coupled receptor / G protein coupled receptor /  Neurokinin / Neurokinin /  Cryo-EM / Peptide Agonists / Cryo-EM / Peptide Agonists /  MEMBRANE PROTEIN MEMBRANE PROTEIN | |||||||||
| Function / homology |  Function and homology information Function and homology information tachykinin receptor activity / positive regulation of flagellated sperm motility / Tachykinin receptors bind tachykinins / positive regulation of uterine smooth muscle contraction / tachykinin receptor signaling pathway / tachykinin receptor activity / positive regulation of flagellated sperm motility / Tachykinin receptors bind tachykinins / positive regulation of uterine smooth muscle contraction / tachykinin receptor signaling pathway /  regulation of feeding behavior / regulation of dopamine metabolic process / positive regulation of blood pressure / G-protein activation / Activation of the phototransduction cascade ... regulation of feeding behavior / regulation of dopamine metabolic process / positive regulation of blood pressure / G-protein activation / Activation of the phototransduction cascade ... tachykinin receptor activity / positive regulation of flagellated sperm motility / Tachykinin receptors bind tachykinins / positive regulation of uterine smooth muscle contraction / tachykinin receptor signaling pathway / tachykinin receptor activity / positive regulation of flagellated sperm motility / Tachykinin receptors bind tachykinins / positive regulation of uterine smooth muscle contraction / tachykinin receptor signaling pathway /  regulation of feeding behavior / regulation of dopamine metabolic process / positive regulation of blood pressure / G-protein activation / Activation of the phototransduction cascade / : / Glucagon-type ligand receptors / Thromboxane signalling through TP receptor / Sensory perception of sweet, bitter, and umami (glutamate) taste / G beta:gamma signalling through PI3Kgamma / G beta:gamma signalling through CDC42 / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / Ca2+ pathway / Activation of G protein gated Potassium channels / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G alpha (z) signalling events / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / Adrenaline,noradrenaline inhibits insulin secretion / ADP signalling through P2Y purinoceptor 12 / G alpha (q) signalling events / G alpha (i) signalling events / Thrombin signalling through proteinase activated receptors (PARs) / Activation of G protein gated Potassium channels / G-protein activation / G beta:gamma signalling through PI3Kgamma / Prostacyclin signalling through prostacyclin receptor / G beta:gamma signalling through PLC beta / ADP signalling through P2Y purinoceptor 1 / Thromboxane signalling through TP receptor / Presynaptic function of Kainate receptors / G beta:gamma signalling through CDC42 / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Glucagon-type ligand receptors / regulation of feeding behavior / regulation of dopamine metabolic process / positive regulation of blood pressure / G-protein activation / Activation of the phototransduction cascade / : / Glucagon-type ligand receptors / Thromboxane signalling through TP receptor / Sensory perception of sweet, bitter, and umami (glutamate) taste / G beta:gamma signalling through PI3Kgamma / G beta:gamma signalling through CDC42 / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / Ca2+ pathway / Activation of G protein gated Potassium channels / Synthesis, secretion, and inactivation of Glucagon-like Peptide-1 (GLP-1) / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / G alpha (z) signalling events / Vasopressin regulates renal water homeostasis via Aquaporins / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / Adrenaline,noradrenaline inhibits insulin secretion / ADP signalling through P2Y purinoceptor 12 / G alpha (q) signalling events / G alpha (i) signalling events / Thrombin signalling through proteinase activated receptors (PARs) / Activation of G protein gated Potassium channels / G-protein activation / G beta:gamma signalling through PI3Kgamma / Prostacyclin signalling through prostacyclin receptor / G beta:gamma signalling through PLC beta / ADP signalling through P2Y purinoceptor 1 / Thromboxane signalling through TP receptor / Presynaptic function of Kainate receptors / G beta:gamma signalling through CDC42 / Inhibition of voltage gated Ca2+ channels via Gbeta/gamma subunits / Glucagon-type ligand receptors /  alkylglycerophosphoethanolamine phosphodiesterase activity / Adrenaline,noradrenaline inhibits insulin secretion / G alpha (12/13) signalling events / G beta:gamma signalling through BTK / ADP signalling through P2Y purinoceptor 12 / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / Thrombin signalling through proteinase activated receptors (PARs) / Ca2+ pathway / G alpha (z) signalling events / Extra-nuclear estrogen signaling / G alpha (s) signalling events / G alpha (q) signalling events / photoreceptor outer segment membrane / G alpha (i) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / neuronal cell body membrane / alkylglycerophosphoethanolamine phosphodiesterase activity / Adrenaline,noradrenaline inhibits insulin secretion / G alpha (12/13) signalling events / G beta:gamma signalling through BTK / ADP signalling through P2Y purinoceptor 12 / Cooperation of PDCL (PhLP1) and TRiC/CCT in G-protein beta folding / Thrombin signalling through proteinase activated receptors (PARs) / Ca2+ pathway / G alpha (z) signalling events / Extra-nuclear estrogen signaling / G alpha (s) signalling events / G alpha (q) signalling events / photoreceptor outer segment membrane / G alpha (i) signalling events / Glucagon-like Peptide-1 (GLP1) regulates insulin secretion / neuronal cell body membrane /  spectrin binding / positive regulation of heart rate / Vasopressin regulates renal water homeostasis via Aquaporins / photoreceptor outer segment / cardiac muscle cell apoptotic process / sperm midpiece / dendrite membrane / photoreceptor inner segment / response to cocaine / cellular response to catecholamine stimulus / adenylate cyclase-activating dopamine receptor signaling pathway / cellular response to prostaglandin E stimulus / sensory perception of taste / G-protein beta-subunit binding / spectrin binding / positive regulation of heart rate / Vasopressin regulates renal water homeostasis via Aquaporins / photoreceptor outer segment / cardiac muscle cell apoptotic process / sperm midpiece / dendrite membrane / photoreceptor inner segment / response to cocaine / cellular response to catecholamine stimulus / adenylate cyclase-activating dopamine receptor signaling pathway / cellular response to prostaglandin E stimulus / sensory perception of taste / G-protein beta-subunit binding /  heterotrimeric G-protein complex / signaling receptor complex adaptor activity / response to estradiol / retina development in camera-type eye / heterotrimeric G-protein complex / signaling receptor complex adaptor activity / response to estradiol / retina development in camera-type eye /  GTPase binding / GTPase binding /  cell body / phospholipase C-activating G protein-coupled receptor signaling pathway / positive regulation of cytosolic calcium ion concentration / cellular response to hypoxia / G alpha (q) signalling events / cell population proliferation / G protein-coupled receptor signaling pathway / cell body / phospholipase C-activating G protein-coupled receptor signaling pathway / positive regulation of cytosolic calcium ion concentration / cellular response to hypoxia / G alpha (q) signalling events / cell population proliferation / G protein-coupled receptor signaling pathway /  GTPase activity / GTPase activity /  dendrite / protein-containing complex binding / dendrite / protein-containing complex binding /  membrane / membrane /  plasma membrane / plasma membrane /  cytoplasm cytoplasmSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens (human) / Homo sapiens (human) /   Rattus norvegicus (Norway rat) / Rattus norvegicus (Norway rat) /   Mus musculoides (Temminck's mouse) / Mus musculoides (Temminck's mouse) /   Bos taurus (cattle) Bos taurus (cattle) | |||||||||
| Method |  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.0 Å cryo EM / Resolution: 3.0 Å | |||||||||
 Authors Authors | Sun WJ / Yang F / Zhang HH / Yuan QN / Yin WC / Shi P / Eric X / Tian CL | |||||||||
| Funding support |  China, 1 items China, 1 items
| |||||||||
 Citation Citation |  Journal: Cell Discov / Year: 2023 Journal: Cell Discov / Year: 2023Title: Structural insights into neurokinin 3 receptor activation by endogenous and analogue peptide agonists. Authors: Wenjing Sun / Fan Yang / Huanhuan Zhang / Qingning Yuan / Shenglong Ling / Yuanxia Wang / Pei Lv / Zelin Li / Yifan Luo / Dongsheng Liu / Wanchao Yin / Pan Shi / H Eric Xu / Changlin Tian /  Abstract: Neurokinin 3 receptor (NK3R) is a tachykinin receptor essential for the hypothalamic-pituitary-gonadal axis. The endogenous peptide agonist neurokinin B (NKB) preferentially activates NK3R, while ...Neurokinin 3 receptor (NK3R) is a tachykinin receptor essential for the hypothalamic-pituitary-gonadal axis. The endogenous peptide agonist neurokinin B (NKB) preferentially activates NK3R, while substance P (SP) binds preferentially to NK1R. In addition, the SP analogue senktide more potently activates NK3R than NKB and SP. However, the mechanisms of preferential binding of peptide and NK3R activation remain elusive. Herein, we determined the cryogenic electron microscopy (cryo-EM) structures of the NK3R-G complex bound to NKB, SP and senktide. The three NK3R-G/peptide complexes utilize a class of noncanonical receptor activation mechanisms. Combining the structural analysis and functional assay illustrated that the consensus C-termini of the three peptide agonists share a conserved binding mode to NK3R, while the divergent N-termini of the peptides confer the preferential binding of the agonist to NK3R. In addition, the specific interactions between the N-terminus of senktide and the N-terminus and extracellular loops (ECL2 and ECL3) of NK3R lead to the improved activation displayed by senktide compared to SP and NKB. These findings pave the way to understand tachykinin receptor subtype selectivity and provide ideas to rationally develop drugs targeting NK3R. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Supplemental images |
|---|
- Downloads & links
Downloads & links
-EMDB archive
| Map data |  emd_36144.map.gz emd_36144.map.gz | 56.9 MB |  EMDB map data format EMDB map data format | |
|---|---|---|---|---|
| Header (meta data) |  emd-36144-v30.xml emd-36144-v30.xml emd-36144.xml emd-36144.xml | 20.6 KB 20.6 KB | Display Display |  EMDB header EMDB header |
| Images |  emd_36144.png emd_36144.png | 147.3 KB | ||
| Filedesc metadata |  emd-36144.cif.gz emd-36144.cif.gz | 6.6 KB | ||
| Others |  emd_36144_half_map_1.map.gz emd_36144_half_map_1.map.gz emd_36144_half_map_2.map.gz emd_36144_half_map_2.map.gz | 49.6 MB 49.6 MB | ||
| Archive directory |  http://ftp.pdbj.org/pub/emdb/structures/EMD-36144 http://ftp.pdbj.org/pub/emdb/structures/EMD-36144 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36144 ftp://ftp.pdbj.org/pub/emdb/structures/EMD-36144 | HTTPS FTP |
-Related structure data
| Related structure data |  8jbfMC  8jbgC  8jbhC M: atomic model generated by this map C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
| EMDB pages |  EMDB (EBI/PDBe) / EMDB (EBI/PDBe) /  EMDataResource EMDataResource |
|---|---|
| Related items in Molecule of the Month |
- Map
Map
| File |  Download / File: emd_36144.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) Download / File: emd_36144.map.gz / Format: CCP4 / Size: 64 MB / Type: IMAGE STORED AS FLOATING POINT NUMBER (4 BYTES) | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Senktide bound to active human neurokinin 3 receptor in complex with Gq | ||||||||||||||||||||
| Voxel size | X=Y=Z: 1.03 Å | ||||||||||||||||||||
| Density |
| ||||||||||||||||||||
| Symmetry | Space group: 1 | ||||||||||||||||||||
| Details | EMDB XML:
|
-Supplemental data
-Half map: Senktide bound to active human neurokinin 3 receptor...
| File | emd_36144_half_map_1.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Senktide bound to active human neurokinin 3 receptor in complex with Gq | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
-Half map: Senktide bound to active human neurokinin 3 receptor...
| File | emd_36144_half_map_2.map | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Annotation | Senktide bound to active human neurokinin 3 receptor in complex with Gq | ||||||||||||
| Projections & Slices |
| ||||||||||||
| Density Histograms |
- Sample components
Sample components
-Entire : Senktide bound to active human neurokinin 3 receptor in complex w...
| Entire | Name: Senktide bound to active human neurokinin 3 receptor in complex with Gq heterotrimer |
|---|---|
| Components |
|
-Supramolecule #1: Senktide bound to active human neurokinin 3 receptor in complex w...
| Supramolecule | Name: Senktide bound to active human neurokinin 3 receptor in complex with Gq heterotrimer type: complex / ID: 1 / Parent: 0 / Macromolecule list: all |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
-Macromolecule #1: Neuromedin-K receptor
| Macromolecule | Name: Neuromedin-K receptor / type: protein_or_peptide / ID: 1 / Details: NK3R-pFastbac1 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 44.31702 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MATLPAAETW IDGGGGVGAD AVNLTASLAA GAATGAVETG WLQLLDQAGN LSSSPSALGL PVASPAPSQP WANLTNQFVQ PSWRIALWS LAYGVVVAVA VLGNLIVIWI ILAHKRMRTV TNYFLVNLAF SDASMAAFNT LVNFIYALHS EWYFGANYCR F QNFFPITA ...String: MATLPAAETW IDGGGGVGAD AVNLTASLAA GAATGAVETG WLQLLDQAGN LSSSPSALGL PVASPAPSQP WANLTNQFVQ PSWRIALWS LAYGVVVAVA VLGNLIVIWI ILAHKRMRTV TNYFLVNLAF SDASMAAFNT LVNFIYALHS EWYFGANYCR F QNFFPITA VFASIYSMTA IAVDRYMAII DPLKPRLSAT ATKIVIGSIW ILAFLLAFPQ CLYSKTKVMP GRTLCFVQWP EG PKQHFTY HIIVIILVYC FPLLIMGITY TIVGITLWGG EIPGDTCDKY HEQLKAKRKV VKMMIIVVMT FAICWLPYHI YFI LTAIYQ QLNRWKYIQQ VYLASFWLAM SSTMYNPIIY CCLNKRFRAG FKRAFRWCPF IKVSSYDELE LKTTRFHP UniProtKB: Neuromedin-K receptor |
-Macromolecule #2: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 type: protein_or_peptide / ID: 2 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Rattus norvegicus (Norway rat) Rattus norvegicus (Norway rat) |
| Molecular weight | Theoretical: 41.042828 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: HMHHHHHGSL LQSELDQLRQ EAEQLKNQIR DARKACADAT LSQITNNIDP VGRIQMRTRR TLRGHLAKIY AMHWGTDSRL LVSASQDGK LIIWDSYTTN KVHAIPLRSS WVMTCAYAPS GNYVACGGLD NICSIYNLKT RDGDVRVSRE LAGHTGYLSC C RFLDDNQI ...String: HMHHHHHGSL LQSELDQLRQ EAEQLKNQIR DARKACADAT LSQITNNIDP VGRIQMRTRR TLRGHLAKIY AMHWGTDSRL LVSASQDGK LIIWDSYTTN KVHAIPLRSS WVMTCAYAPS GNYVACGGLD NICSIYNLKT RDGDVRVSRE LAGHTGYLSC C RFLDDNQI VTSSGDTTCA LWDIETGQQT TTFTGHTGDV MSLSLAPDTR LFVSGACDAS AKLWDVREGM CRQTFTGHES DI NAICFFP NGNAFATGSD DATCRLFDLR ADQELMTYSH DNIICGITSV SFSKSGRLLL AGYDDFNCNV WDALKADRAG VLA GHDNRV SCLGVTDDGM AVATGSWDSF LKIWNGSSGG GGSGGGGSSG VSGWRLFKKI S UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(T) subunit beta-1 |
-Macromolecule #3: ScFv16 nanobody
| Macromolecule | Name: ScFv16 nanobody / type: protein_or_peptide / ID: 3 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Mus musculoides (Temminck's mouse) Mus musculoides (Temminck's mouse) |
| Molecular weight | Theoretical: 32.708473 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MLLVNQSHQG FNKEHTSKMV SAIVLYVLLA AAAHSAFAVQ LVESGGGLVQ PGGSRKLSCS ASGFAFSSFG MHWVRQAPEK GLEWVAYIS SGSGTIYYAD TVKGRFTISR DDPKNTLFLQ MTSLRSEDTA MYYCVRSIYY YGSSPFDFWG QGTTLTVSAG G GGSGGGGS ...String: MLLVNQSHQG FNKEHTSKMV SAIVLYVLLA AAAHSAFAVQ LVESGGGLVQ PGGSRKLSCS ASGFAFSSFG MHWVRQAPEK GLEWVAYIS SGSGTIYYAD TVKGRFTISR DDPKNTLFLQ MTSLRSEDTA MYYCVRSIYY YGSSPFDFWG QGTTLTVSAG G GGSGGGGS GGGGSSDIVM TQATSSVPVT PGESVSISCR SSKSLLHSNG NTYLYWFLQR PGQSPQLLIY RMSNLASGVP DR FSGSGSG TAFTLTISRL EAEDVGVYYC MQHLEYPLTF GAGTKLELVD ENLYFQGASH HHHHHHH |
-Macromolecule #4: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2
| Macromolecule | Name: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 type: protein_or_peptide / ID: 4 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Bos taurus (cattle) Bos taurus (cattle) |
| Molecular weight | Theoretical: 7.861143 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MASNNTASIA QARKLVEQLK MEANIDRIKV SKAAADLMAY CEAHAKEDPL LTPVPASENP FREKKFFCAI L UniProtKB: Guanine nucleotide-binding protein G(I)/G(S)/G(O) subunit gamma-2 |
-Macromolecule #5: Senktide
| Macromolecule | Name: Senktide / type: protein_or_peptide / ID: 5 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 915.022 Da |
| Recombinant expression | Organism:   Escherichia coli (E. coli) Escherichia coli (E. coli) |
| Sequence | String: DDFFGLMA |
-Macromolecule #6: Guanine nucleotide-binding protein Gq subunit alpha
| Macromolecule | Name: Guanine nucleotide-binding protein Gq subunit alpha / type: protein_or_peptide / ID: 6 / Number of copies: 1 / Enantiomer: LEVO |
|---|---|
| Source (natural) | Organism:   Homo sapiens (human) Homo sapiens (human) |
| Molecular weight | Theoretical: 41.694289 KDa |
| Recombinant expression | Organism:   Spodoptera frugiperda (fall armyworm) Spodoptera frugiperda (fall armyworm) |
| Sequence | String: MGCTLSAEDK AAVERSKMIE KQLQKDKQVY RRTLRLLLLG ADNSGKSTIV KQMRIYHVNG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI ...String: MGCTLSAEDK AAVERSKMIE KQLQKDKQVY RRTLRLLLLG ADNSGKSTIV KQMRIYHVNG YSEEECKQYK AVVYSNTIQS IIAIIRAMG RLKIDFGDSA RADDARQLFV LAGAAEEGFM TAELAGVIKR LWKDSGVQAC FNRSREYQLN DSAAYYLNDL D RIAQPNYI PTQQDVLRTR VKTSGIFETK FQVDKVNFHM FDVGAQRDER RKWIQCFNDV TAIIFVVDSS DYNRLQEALN DF DSIWNNR WLRTISVILF LNKQDLLAEK VLAGKSKIED YFPEFARYTT PEDATPEPGE DPRVTRAKYF IRKEFVDIST ASG DGRHIC YPHFTCAVDT ENARRIFNDC KDIILQMNLR EYNLV |
-Experimental details
-Structure determination
| Method |  cryo EM cryo EM |
|---|---|
 Processing Processing |  single particle reconstruction single particle reconstruction |
| Aggregation state | particle |
- Sample preparation
Sample preparation
| Concentration | 1.0 mg/mL |
|---|---|
| Buffer | pH: 7.5 |
| Vitrification | Cryogen name: ETHANE-PROPANE / Chamber humidity: 100 % |
- Electron microscopy
Electron microscopy
| Microscope | FEI TITAN KRIOS |
|---|---|
| Electron beam | Acceleration voltage: 300 kV / Electron source:  FIELD EMISSION GUN FIELD EMISSION GUN |
| Electron optics | Illumination mode: SPOT SCAN / Imaging mode: DIFFRACTION / Cs: 2.7 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.2 µm / Cs: 2.7 mm / Nominal defocus max: 2.2 µm / Nominal defocus min: 1.2 µm |
| Image recording | Film or detector model: GATAN K3 (6k x 4k) / Average electron dose: 50.0 e/Å2 |
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
- Image processing
Image processing
| Startup model | Type of model: PDB ENTRY PDB model - PDB ID: |
|---|---|
| Initial angle assignment | Type: ANGULAR RECONSTITUTION |
| Final angle assignment | Type: ANGULAR RECONSTITUTION |
| Final reconstruction | Resolution.type: BY AUTHOR / Resolution: 3.0 Å / Resolution method: FSC 0.143 CUT-OFF / Number images used: 2457160 |
 Movie
Movie Controller
Controller


















 Z
Z Y
Y X
X


















