+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8h69 | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
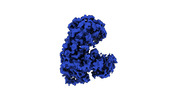
| Title | Cryo-EM structure of influenza RNA polymerase | ||||||||||||
 Components Components |
| ||||||||||||
 Keywords Keywords |  VIRAL PROTEIN / VIRAL PROTEIN /  RNA polymerase RNA polymerase | ||||||||||||
| Function / homology |  Function and homology information Function and homology informationsymbiont-mediated suppression of host mRNA transcription via inhibition of RNA polymerase II activity /  cap snatching / 7-methylguanosine mRNA capping / viral transcription / host cell mitochondrion / cap snatching / 7-methylguanosine mRNA capping / viral transcription / host cell mitochondrion /  virion component / virion component /  endonuclease activity / host cell cytoplasm / endonuclease activity / host cell cytoplasm /  Hydrolases; Acting on ester bonds / Hydrolases; Acting on ester bonds /  RNA-directed RNA polymerase ...symbiont-mediated suppression of host mRNA transcription via inhibition of RNA polymerase II activity / RNA-directed RNA polymerase ...symbiont-mediated suppression of host mRNA transcription via inhibition of RNA polymerase II activity /  cap snatching / 7-methylguanosine mRNA capping / viral transcription / host cell mitochondrion / cap snatching / 7-methylguanosine mRNA capping / viral transcription / host cell mitochondrion /  virion component / virion component /  endonuclease activity / host cell cytoplasm / endonuclease activity / host cell cytoplasm /  Hydrolases; Acting on ester bonds / Hydrolases; Acting on ester bonds /  RNA-directed RNA polymerase / viral RNA genome replication / RNA-directed RNA polymerase / viral RNA genome replication /  RNA-dependent RNA polymerase activity / RNA-dependent RNA polymerase activity /  nucleotide binding / DNA-templated transcription / host cell nucleus / nucleotide binding / DNA-templated transcription / host cell nucleus /  RNA binding / RNA binding /  metal ion binding / metal ion binding /  cytoplasm cytoplasmSimilarity search - Function | ||||||||||||
| Biological species |    Influenza A virus Influenza A virus | ||||||||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.7 Å cryo EM / Resolution: 3.7 Å | ||||||||||||
 Authors Authors | Li, H. / Wu, Y. / Liang, H. / Liu, Y. | ||||||||||||
| Funding support |  China, 3items China, 3items
| ||||||||||||
 Citation Citation |  Journal: Nat Struct Mol Biol / Year: 2023 Journal: Nat Struct Mol Biol / Year: 2023Title: An intermediate state allows influenza polymerase to switch smoothly between transcription and replication cycles. Authors: Huanhuan Li / Yixi Wu / Minke Li / Lu Guo / Yaqi Gao / Quan Wang / Jihua Zhang / Zhaohua Lai / Xing Zhang / Lixin Zhu / Ping Lan / Zihe Rao / Yingfang Liu / Huanhuan Liang /  Abstract: Influenza polymerase (FluPol) transcribes viral mRNA at the beginning of the viral life cycle and initiates genome replication after viral protein synthesis. However, it remains poorly understood how ...Influenza polymerase (FluPol) transcribes viral mRNA at the beginning of the viral life cycle and initiates genome replication after viral protein synthesis. However, it remains poorly understood how FluPol switches between its transcription and replication states, especially given that the structural bases of these two functions are fundamentally different. Here we propose a mechanism by which FluPol achieves functional switching between these two states through a previously unstudied conformation, termed an 'intermediate state'. Using cryo-electron microscopy, we obtained a structure of the intermediate state of H5N1 FluPol at 3.7 Å, which is characterized by a blocked cap-binding domain and a contracted core region. Structural analysis results suggest that the intermediate state may allow FluPol to transition smoothly into either the transcription or replication state. Furthermore, we show that the formation of the intermediate state is required for both the transcription and replication activities of FluPol, leading us to conclude that the transcription and replication cycles of FluPol are regulated via this intermediate state. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8h69.cif.gz 8h69.cif.gz | 406.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8h69.ent.gz pdb8h69.ent.gz | 324.3 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8h69.json.gz 8h69.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/h6/8h69 https://data.pdbj.org/pub/pdb/validation_reports/h6/8h69 ftp://data.pdbj.org/pub/pdb/validation_reports/h6/8h69 ftp://data.pdbj.org/pub/pdb/validation_reports/h6/8h69 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  34497MC M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
| #1: RNA chain | Mass: 5608.307 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Influenza A virus (A/goose/Guangdong/1/1996(H5N1)) Influenza A virus (A/goose/Guangdong/1/1996(H5N1))Strain: A/Goose/Guangdong/1/1996 H5N1 genotype Gs/Gd Production host: Insect cell expression vector pTIE1 (others) |
|---|---|
| #2: RNA chain | Mass: 6150.730 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Influenza A virus (A/goose/Guangdong/1/1996(H5N1)) Influenza A virus (A/goose/Guangdong/1/1996(H5N1))Strain: A/Goose/Guangdong/1/1996 H5N1 genotype Gs/Gd Production host: Insect cell expression vector pTIE1 (others) |
| #3: Protein | Mass: 82669.469 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Influenza A virus (A/goose/Guangdong/1/1996(H5N1)) Influenza A virus (A/goose/Guangdong/1/1996(H5N1))Strain: A/Goose/Guangdong/1/1996 H5N1 genotype Gs/Gd / Gene: PA Production host: Insect cell expression vector pTIE1 (others) References: UniProt: Q91R78 |
| #4: Protein | Mass: 86492.195 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Influenza A virus (A/goose/Guangdong/1/1996(H5N1)) Influenza A virus (A/goose/Guangdong/1/1996(H5N1))Strain: A/Goose/Guangdong/1/1996 H5N1 genotype Gs/Gd / Gene: PB1 Production host: Insect cell expression vector pTIE1 (others) References: UniProt: Q91HA4 |
| #5: Protein | Mass: 85954.047 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Influenza A virus (A/goose/Guangdong/1/1996(H5N1)) Influenza A virus (A/goose/Guangdong/1/1996(H5N1))Strain: A/Goose/Guangdong/1/1996 H5N1 genotype Gs/Gd / Gene: PB2 Production host: Insect cell expression vector pTIE1 (others) References: UniProt: Q6E3M8 |
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: polymerase / Type: COMPLEX / Entity ID: all / Source: RECOMBINANT / Type: COMPLEX / Entity ID: all / Source: RECOMBINANT |
|---|---|
| Source (natural) | Organism:    Influenza A virus Influenza A virus |
| Source (recombinant) | Organism: Insect cell expression vector pTIE1 (others) |
| Buffer solution | pH: 7.8 |
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YES |
Vitrification | Cryogen name: ETHANE |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: DARK FIELD / Nominal defocus max: 2500 nm / Nominal defocus min: 1500 nm |
| Image recording | Electron dose: 10 e/Å2 / Film or detector model: GATAN K2 SUMMIT (4k x 4k) |
- Processing
Processing
CTF correction | Type: PHASE FLIPPING AND AMPLITUDE CORRECTION |
|---|---|
3D reconstruction | Resolution: 3.7 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 790730 / Symmetry type: POINT |
 Movie
Movie Controller
Controller



 PDBj
PDBj






























