+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 8giy | ||||||
|---|---|---|---|---|---|---|---|
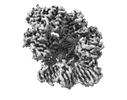
| Title | E. coli clamp loader with closed clamp | ||||||
 Components Components |
| ||||||
 Keywords Keywords | DNA BINDING PROTEIN/DNA / clamp loader /  DNA clamp / DNA clamp /  AAA+ / AAA+ /  ATPase / DNA BINDING PROTEIN-DNA complex ATPase / DNA BINDING PROTEIN-DNA complex | ||||||
| Function / homology |  Function and homology information Function and homology information DNA polymerase III, clamp loader complex / Hda-beta clamp complex / bacterial-type DNA replication / replication inhibiting complex / DNA polymerase III, clamp loader complex / Hda-beta clamp complex / bacterial-type DNA replication / replication inhibiting complex /  DNA polymerase III complex / DNA polymerase III complex /  replisome / regulation of DNA-templated DNA replication initiation / DNA strand elongation involved in DNA replication / DNA polymerase processivity factor activity / error-prone translesion synthesis ... replisome / regulation of DNA-templated DNA replication initiation / DNA strand elongation involved in DNA replication / DNA polymerase processivity factor activity / error-prone translesion synthesis ... DNA polymerase III, clamp loader complex / Hda-beta clamp complex / bacterial-type DNA replication / replication inhibiting complex / DNA polymerase III, clamp loader complex / Hda-beta clamp complex / bacterial-type DNA replication / replication inhibiting complex /  DNA polymerase III complex / DNA polymerase III complex /  replisome / regulation of DNA-templated DNA replication initiation / DNA strand elongation involved in DNA replication / DNA polymerase processivity factor activity / error-prone translesion synthesis / negative regulation of DNA-templated DNA replication initiation / 3'-5' exonuclease activity / ribonucleoside triphosphate phosphatase activity / response to radiation / DNA-templated DNA replication / replisome / regulation of DNA-templated DNA replication initiation / DNA strand elongation involved in DNA replication / DNA polymerase processivity factor activity / error-prone translesion synthesis / negative regulation of DNA-templated DNA replication initiation / 3'-5' exonuclease activity / ribonucleoside triphosphate phosphatase activity / response to radiation / DNA-templated DNA replication /  DNA replication / DNA replication /  DNA-directed DNA polymerase / DNA-directed DNA polymerase /  DNA-directed DNA polymerase activity / DNA damage response / DNA-directed DNA polymerase activity / DNA damage response /  ATP hydrolysis activity / protein homodimerization activity / ATP hydrolysis activity / protein homodimerization activity /  DNA binding / DNA binding /  ATP binding / identical protein binding / ATP binding / identical protein binding /  cytosol cytosolSimilarity search - Function | ||||||
| Biological species |   Escherichia coli K-12 (bacteria) Escherichia coli K-12 (bacteria) | ||||||
| Method |  ELECTRON MICROSCOPY / ELECTRON MICROSCOPY /  single particle reconstruction / single particle reconstruction /  cryo EM / Resolution: 3.7 Å cryo EM / Resolution: 3.7 Å | ||||||
 Authors Authors | Oakley, A.J. / Xu, Z.-Q. / Dixon, N.E. | ||||||
| Funding support |  Australia, 1items Australia, 1items
| ||||||
 Citation Citation |  Journal: To Be Published Journal: To Be PublishedTitle: Structural characterisation of the complete cycle of sliding clamp loading in E. coli Authors: Xu, Z.-Q. / Oakley, A.J. / Dixon, N.E. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  8giy.cif.gz 8giy.cif.gz | 992.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb8giy.ent.gz pdb8giy.ent.gz | 688.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  8giy.json.gz 8giy.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/gi/8giy https://data.pdbj.org/pub/pdb/validation_reports/gi/8giy ftp://data.pdbj.org/pub/pdb/validation_reports/gi/8giy ftp://data.pdbj.org/pub/pdb/validation_reports/gi/8giy | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  40079MC  8gizC  8gj0C  8gj1C  8gj2C  8gj3C M: map data used to model this data C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
|
|---|---|
| 1 |
|
- Components
Components
-DNA polymerase III subunit ... , 4 types, 6 molecules ABCDEF
| #1: Protein |  DNA polymerase III holoenzyme DNA polymerase III holoenzymeMass: 38745.574 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Escherichia coli K-12 (bacteria) / Gene: holA, b0640, JW0635 / Production host: Escherichia coli K-12 (bacteria) / Gene: holA, b0640, JW0635 / Production host:   Escherichia coli BL21 (bacteria) / References: UniProt: P28630, Escherichia coli BL21 (bacteria) / References: UniProt: P28630,  DNA-directed DNA polymerase DNA-directed DNA polymerase | ||||
|---|---|---|---|---|---|
| #2: Protein |  DNA polymerase III holoenzyme / DNA polymerase III subunit gamma DNA polymerase III holoenzyme / DNA polymerase III subunit gammaMass: 47601.766 Da / Num. of mol.: 3 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Escherichia coli K-12 (bacteria) / Gene: dnaX, dnaZ, dnaZX, b0470, JW0459 / Production host: Escherichia coli K-12 (bacteria) / Gene: dnaX, dnaZ, dnaZX, b0470, JW0459 / Production host:   Escherichia coli BL21 (bacteria) / References: UniProt: P06710, Escherichia coli BL21 (bacteria) / References: UniProt: P06710,  DNA-directed DNA polymerase DNA-directed DNA polymerase#3: Protein | |  DNA polymerase III holoenzyme DNA polymerase III holoenzymeMass: 36980.484 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Escherichia coli K-12 (bacteria) / Gene: holB, b1099, JW1085 / Production host: Escherichia coli K-12 (bacteria) / Gene: holB, b1099, JW1085 / Production host:   Escherichia coli BL21 (bacteria) / References: UniProt: P28631, Escherichia coli BL21 (bacteria) / References: UniProt: P28631,  DNA-directed DNA polymerase DNA-directed DNA polymerase#4: Protein | |  DNA polymerase III holoenzyme DNA polymerase III holoenzymeMass: 15188.276 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Escherichia coli K-12 (bacteria) / Gene: holD, b4372, JW4334 / Production host: Escherichia coli K-12 (bacteria) / Gene: holD, b4372, JW4334 / Production host:   Escherichia coli BL21 (bacteria) / References: UniProt: P28632, Escherichia coli BL21 (bacteria) / References: UniProt: P28632,  DNA-directed DNA polymerase DNA-directed DNA polymerase |
-Protein , 1 types, 2 molecules HI
| #5: Protein | Mass: 40630.508 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Escherichia coli K-12 (bacteria) / Gene: dnaN, b3701, JW3678 / Production host: Escherichia coli K-12 (bacteria) / Gene: dnaN, b3701, JW3678 / Production host:   Escherichia coli BL21 (bacteria) / References: UniProt: P0A988 Escherichia coli BL21 (bacteria) / References: UniProt: P0A988 |
|---|
-Non-polymers , 3 types, 10 molecules 




| #6: Chemical | | #7: Chemical | #8: Chemical | ChemComp-ZN / |
|---|
-Details
| Has ligand of interest | N |
|---|
-Experimental details
-Experiment
| Experiment | Method:  ELECTRON MICROSCOPY ELECTRON MICROSCOPY |
|---|---|
| EM experiment | Aggregation state: PARTICLE / 3D reconstruction method:  single particle reconstruction single particle reconstruction |
- Sample preparation
Sample preparation
| Component | Name: E. coli clamp loader with closed clamp / Type: COMPLEX Details: Clamp loader complex composed of DNA polymerase III delta gamma(3) delta' chi and psi subunits. Clamp composed of DNA polymerase III beta(2) subunits. Entity ID: #1-#5 / Source: RECOMBINANT | |||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Molecular weight | Value: 0.331 MDa / Experimental value: NO | |||||||||||||||||||||||||||||||||||
| Source (natural) | Organism:   Escherichia coli (E. coli) / Strain: K-12 Escherichia coli (E. coli) / Strain: K-12 | |||||||||||||||||||||||||||||||||||
| Source (recombinant) | Organism:   Escherichia coli (E. coli) / Strain: BL21 Escherichia coli (E. coli) / Strain: BL21 | |||||||||||||||||||||||||||||||||||
| Buffer solution | pH: 7.5 Details: 30 mM Na.HEPES pH 7.5, 3 mM MgCl2, 2 mM dithiothreitol, 0.25 mM EDTA, 2% glycerol, 1 mM ATPgammaS. | |||||||||||||||||||||||||||||||||||
| Buffer component |
| |||||||||||||||||||||||||||||||||||
| Specimen | Embedding applied: NO / Shadowing applied: NO / Staining applied : NO / Vitrification applied : NO / Vitrification applied : YES : YESDetails: 30 microL of 6 microM delta/gamma3/delta' mixed with chi/psi complex at a molar ratio of 1:1.2, beta2 at 1:1.3, and dialysed twice at 4 degrees C against 250 mL of 30 mM Na.HEPES pH 7.5, 3 ...Details: 30 microL of 6 microM delta/gamma3/delta' mixed with chi/psi complex at a molar ratio of 1:1.2, beta2 at 1:1.3, and dialysed twice at 4 degrees C against 250 mL of 30 mM Na.HEPES pH 7.5, 3 mM MgCl2, 2 mM dithiothreitol, 0.25 mM EDTA, 2% glycerol. 1 mM ATPgammaS was added to the dialysate. | |||||||||||||||||||||||||||||||||||
| Specimen support | Details: Use a Zepto Low-pressure plasma systems (PLASMA CLEANER) (Diener Electronic) at 10% power for 120 seconds. Grid material: GOLD / Grid type: Quantifoil R1.2/1.3 | |||||||||||||||||||||||||||||||||||
Vitrification | Instrument: FEI VITROBOT MARK IV / Cryogen name: ETHANE / Humidity: 100 % / Chamber temperature: 279 K |
- Electron microscopy imaging
Electron microscopy imaging
| Experimental equipment |  Model: Titan Krios / Image courtesy: FEI Company |
|---|---|
| Microscopy | Model: FEI TITAN KRIOS |
| Electron gun | Electron source : :  FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM FIELD EMISSION GUN / Accelerating voltage: 300 kV / Illumination mode: FLOOD BEAM |
| Electron lens | Mode: BRIGHT FIELD Bright-field microscopy / Nominal magnification: 165000 X / Nominal defocus max: 1300 nm / Nominal defocus min: 400 nm / Cs Bright-field microscopy / Nominal magnification: 165000 X / Nominal defocus max: 1300 nm / Nominal defocus min: 400 nm / Cs : 2.7 mm / C2 aperture diameter: 70 µm / Alignment procedure: COMA FREE : 2.7 mm / C2 aperture diameter: 70 µm / Alignment procedure: COMA FREE |
| Specimen holder | Cryogen: NITROGEN / Specimen holder model: FEI TITAN KRIOS AUTOGRID HOLDER |
| Image recording | Average exposure time: 6 sec. / Electron dose: 50 e/Å2 / Detector mode: COUNTING / Film or detector model: GATAN K2 SUMMIT (4k x 4k) / Num. of grids imaged: 1 / Num. of real images: 7269 |
| EM imaging optics | Energyfilter name : GIF Bioquantum / Details: unfiltered mode : GIF Bioquantum / Details: unfiltered mode |
| Image scans | Width: 4096 / Height: 4096 / Movie frames/image: 60 / Used frames/image: 1-60 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| EM software |
| ||||||||||||||||||||||||||||||||||||||||
CTF correction | Details: CTF refinement per-particle was performed in RELION before final 3D reconstruction. Type: PHASE FLIPPING AND AMPLITUDE CORRECTION | ||||||||||||||||||||||||||||||||||||||||
| Particle selection | Num. of particles selected: 1376000 | ||||||||||||||||||||||||||||||||||||||||
| Symmetry | Point symmetry : C1 (asymmetric) : C1 (asymmetric) | ||||||||||||||||||||||||||||||||||||||||
3D reconstruction | Resolution: 3.7 Å / Resolution method: FSC 0.143 CUT-OFF / Num. of particles: 373000 / Algorithm: BACK PROJECTION / Num. of class averages: 2 / Symmetry type: POINT | ||||||||||||||||||||||||||||||||||||||||
| Atomic model building | Protocol: RIGID BODY FIT | ||||||||||||||||||||||||||||||||||||||||
| Atomic model building |
| ||||||||||||||||||||||||||||||||||||||||
| Refinement | Cross valid method: NONE Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2 | ||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 271.99 Å2 | ||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller







 PDBj
PDBj





