[English] 日本語
 Yorodumi
Yorodumi- PDB-7t5d: Neutron structure of Neurospora crassa Lytic Polysaccharide Monoo... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7t5d | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Neutron structure of Neurospora crassa Lytic Polysaccharide Monooxygenase 9D (NcLPMO9D) ascorbate soak | ||||||||||||
 Components Components | Lytic polysaccharide monooxygenase | ||||||||||||
 Keywords Keywords |  OXIDOREDUCTASE / LPMO / OXIDOREDUCTASE / LPMO /  monooxygenase / PMO / monooxygenase / PMO /  metalloproteins / metalloproteins /  copper copper | ||||||||||||
| Function / homology |  Function and homology information Function and homology information monooxygenase activity / monooxygenase activity /  hydrolase activity / extracellular region / hydrolase activity / extracellular region /  metal ion binding metal ion bindingSimilarity search - Function | ||||||||||||
| Biological species |   Neurospora crassa (fungus) Neurospora crassa (fungus) | ||||||||||||
| Method |  NEUTRON DIFFRACTION / NEUTRON DIFFRACTION /  MOLECULAR REPLACEMENT / Resolution: 2.4 Å MOLECULAR REPLACEMENT / Resolution: 2.4 Å | ||||||||||||
 Authors Authors | Schroder, G.C. / Meilleur, F. | ||||||||||||
| Funding support |  South Africa, 3items South Africa, 3items
| ||||||||||||
 Citation Citation |  Journal: Chem Sci / Year: 2022 Journal: Chem Sci / Year: 2022Title: Capture of activated dioxygen intermediates at the copper-active site of a lytic polysaccharide monooxygenase. Authors: Schroder, G.C. / O'Dell, W.B. / Webb, S.P. / Agarwal, P.K. / Meilleur, F. #1:  Journal: Acta Crystallogr F Struct Biol Commun / Year: 2021 Journal: Acta Crystallogr F Struct Biol Commun / Year: 2021Title: Preliminary results of neutron and X-ray diffraction data collection on a lytic polysaccharide monooxygenase under reduced and acidic conditions. Authors: Schroder, G.C. / O'Dell, W.B. / Swartz, P.D. / Meilleur, F. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7t5d.cif.gz 7t5d.cif.gz | 193.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7t5d.ent.gz pdb7t5d.ent.gz | 154.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7t5d.json.gz 7t5d.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/t5/7t5d https://data.pdbj.org/pub/pdb/validation_reports/t5/7t5d ftp://data.pdbj.org/pub/pdb/validation_reports/t5/7t5d ftp://data.pdbj.org/pub/pdb/validation_reports/t5/7t5d | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7t5cC  7t5eC  5tkhS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||
| 2 | 
| ||||||||||
| Unit cell |
|
- Components
Components
-Protein / Sugars , 2 types, 4 molecules AB
| #1: Protein | Mass: 23299.104 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Neurospora crassa (fungus) / Gene: G15G9.090, GE21DRAFT_7469 / Production host: Neurospora crassa (fungus) / Gene: G15G9.090, GE21DRAFT_7469 / Production host:   Komagataella pastoris (fungus) / Strain (production host): Superman5 / References: UniProt: Q8WZQ2 Komagataella pastoris (fungus) / Strain (production host): Superman5 / References: UniProt: Q8WZQ2#2: Polysaccharide |  / Mass: 424.401 Da / Num. of mol.: 2 / Mass: 424.401 Da / Num. of mol.: 2Source method: isolated from a genetically manipulated source |
|---|
-Non-polymers , 4 types, 417 molecules 
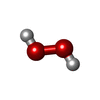





| #3: Chemical |  Copper Copper#4: Chemical | ChemComp-PEO / |  Hydrogen peroxide Hydrogen peroxide#5: Chemical | ChemComp-OXY / |  Oxygen Oxygen#6: Water | ChemComp-HOH / |  Water Water |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  NEUTRON DIFFRACTION / Number of used crystals: 1 NEUTRON DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 1.96 Å3/Da / Density % sol: 37.3 % / Description: Crystals form rectangular shapes. |
|---|---|
Crystal grow | Temperature: 295 K / Method: vapor diffusion / pH: 6 / Details: PEG 3350, HEPES |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source: SPALLATION SOURCE / Site: ORNL Spallation Neutron Source  / Beamline: MANDI / Wavelength: 2.0-4.0 / Beamline: MANDI / Wavelength: 2.0-4.0 | |||||||||
| Detector | Type: ORNL ANGER CAMERA / Detector: DIFFRACTOMETER / Date: Nov 15, 2018 | |||||||||
| Radiation | Protocol: LAUE / Monochromatic (M) / Laue (L): L / Scattering type: neutron | |||||||||
| Radiation wavelength |
| |||||||||
| Reflection | Resolution: 2.4→14.791 Å / Num. obs: 14168 / % possible obs: 91.16 % / Redundancy: 3.2 % / Biso Wilson estimate: 31.17 Å2 / CC1/2: 0.956 / Rmerge(I) obs: 0.1851 / Rrim(I) all: 0.2148 / Net I/σ(I): 7.15 | |||||||||
| Reflection shell | Resolution: 2.4→2.49 Å / Redundancy: 2.3 % / Rmerge(I) obs: 0.2737 / Mean I/σ(I) obs: 2.28 / Num. unique obs: 1300 / CC1/2: 0.297 / Rrim(I) all: 0.3327 / % possible all: 84.86 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 5TKH Resolution: 2.4→14.791 Å / SU ML: 0.41 / Cross valid method: FREE R-VALUE / σ(F): 2.34 / Phase error: 31.99 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 55.48 Å2 / Biso mean: 38.9543 Å2 / Biso min: 21.65 Å2 | ||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.4→14.79 Å
| ||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: NEUTRON DIFFRACTION / Rfactor Rfree error: 0 / Total num. of bins used: 5
|
 Movie
Movie Controller
Controller


 PDBj
PDBj








