[English] 日本語
 Yorodumi
Yorodumi- PDB-7fee: Crystal structure of the allosteric modulator ZCZ011 binding to C... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 7fee | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of the allosteric modulator ZCZ011 binding to CP55940-bound cannabinoid receptor 1 | ||||||
 Components Components | Cannabinoid receptor 1,GlgA glycogen synthase | ||||||
 Keywords Keywords |  MEMBRANE PROTEIN / MEMBRANE PROTEIN /  signal protein / signal protein /  GPCR GPCR | ||||||
| Function / homology |  Function and homology information Function and homology informationcannabinoid signaling pathway /  regulation of penile erection / retrograde trans-synaptic signaling by endocannabinoid / regulation of penile erection / retrograde trans-synaptic signaling by endocannabinoid /  cannabinoid receptor activity / negative regulation of mast cell activation / trans-synaptic signaling by endocannabinoid, modulating synaptic transmission / glycogen (starch) synthase activity / negative regulation of fatty acid beta-oxidation / negative regulation of dopamine secretion / positive regulation of acute inflammatory response to antigenic stimulus ...cannabinoid signaling pathway / cannabinoid receptor activity / negative regulation of mast cell activation / trans-synaptic signaling by endocannabinoid, modulating synaptic transmission / glycogen (starch) synthase activity / negative regulation of fatty acid beta-oxidation / negative regulation of dopamine secretion / positive regulation of acute inflammatory response to antigenic stimulus ...cannabinoid signaling pathway /  regulation of penile erection / retrograde trans-synaptic signaling by endocannabinoid / regulation of penile erection / retrograde trans-synaptic signaling by endocannabinoid /  cannabinoid receptor activity / negative regulation of mast cell activation / trans-synaptic signaling by endocannabinoid, modulating synaptic transmission / glycogen (starch) synthase activity / negative regulation of fatty acid beta-oxidation / negative regulation of dopamine secretion / positive regulation of acute inflammatory response to antigenic stimulus / cannabinoid receptor activity / negative regulation of mast cell activation / trans-synaptic signaling by endocannabinoid, modulating synaptic transmission / glycogen (starch) synthase activity / negative regulation of fatty acid beta-oxidation / negative regulation of dopamine secretion / positive regulation of acute inflammatory response to antigenic stimulus /  regulation of feeding behavior / negative regulation of serotonin secretion / regulation of presynaptic cytosolic calcium ion concentration / negative regulation of action potential / Class A/1 (Rhodopsin-like receptors) / positive regulation of blood pressure / regulation of feeding behavior / negative regulation of serotonin secretion / regulation of presynaptic cytosolic calcium ion concentration / negative regulation of action potential / Class A/1 (Rhodopsin-like receptors) / positive regulation of blood pressure /  regulation of metabolic process / positive regulation of fever generation / axonal fasciculation / regulation of metabolic process / positive regulation of fever generation / axonal fasciculation /  regulation of synaptic transmission, GABAergic / regulation of insulin secretion / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / GABA-ergic synapse / maternal process involved in female pregnancy / regulation of synaptic transmission, GABAergic / regulation of insulin secretion / G protein-coupled receptor signaling pathway, coupled to cyclic nucleotide second messenger / GABA-ergic synapse / maternal process involved in female pregnancy /  regulation of synaptic transmission, glutamatergic / negative regulation of blood pressure / response to nutrient / response to cocaine / G protein-coupled receptor activity / response to nicotine / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / adenylate cyclase-activating G protein-coupled receptor signaling pathway / regulation of synaptic transmission, glutamatergic / negative regulation of blood pressure / response to nutrient / response to cocaine / G protein-coupled receptor activity / response to nicotine / adenylate cyclase-modulating G protein-coupled receptor signaling pathway / adenylate cyclase-activating G protein-coupled receptor signaling pathway /  memory / positive regulation of neuron projection development / memory / positive regulation of neuron projection development /  actin cytoskeleton / actin cytoskeleton /  glucose homeostasis / glucose homeostasis /  presynaptic membrane / G alpha (i) signalling events / presynaptic membrane / G alpha (i) signalling events /  growth cone / growth cone /  spermatogenesis / response to ethanol / mitochondrial outer membrane / response to lipopolysaccharide / spermatogenesis / response to ethanol / mitochondrial outer membrane / response to lipopolysaccharide /  membrane raft / positive regulation of apoptotic process / membrane raft / positive regulation of apoptotic process /  nucleotide binding / glutamatergic synapse / identical protein binding / nucleotide binding / glutamatergic synapse / identical protein binding /  plasma membrane / plasma membrane /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human)   Pyrococcus abyssi GE5 (archaea) Pyrococcus abyssi GE5 (archaea) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.7 Å MOLECULAR REPLACEMENT / Resolution: 2.7 Å | ||||||
 Authors Authors | Wang, X. / Zhao, C. / Shao, Z. | ||||||
| Funding support | 1items
| ||||||
 Citation Citation |  Journal: Nat Chem Biol / Year: 2022 Journal: Nat Chem Biol / Year: 2022Title: Molecular mechanism of allosteric modulation for the cannabinoid receptor CB1. Authors: Xin Yang / Xuehui Wang / Zheng Xu / Chao Wu / Yangli Zhou / Yifei Wang / Guifeng Lin / Kan Li / Ming Wu / Anjie Xia / Jingming Liu / Lin Cheng / Jun Zou / Wei Yan / Zhenhua Shao / Shengyong Yang /  Abstract: Given the promising clinical value of allosteric modulators of G protein-coupled-receptors (GPCRs), mechanistic understanding of how these modulators alter GPCR function is of significance. Here, we ...Given the promising clinical value of allosteric modulators of G protein-coupled-receptors (GPCRs), mechanistic understanding of how these modulators alter GPCR function is of significance. Here, we report the crystallographic and cryo-electron microscopy structures of the cannabinoid receptor CB1 bound to the positive allosteric modulator (PAM) ZCZ011. These structures show that ZCZ011 binds to an extrahelical site in the transmembrane 2 (TM2)-TM3-TM4 surface. Through (un)biased molecular dynamics simulations and mutagenesis experiments, we show that TM2 rearrangement is critical for the propagation of allosteric signals. ZCZ011 exerts a PAM effect by promoting TM2 rearrangement in favor of receptor activation and increasing the population of receptors that adopt an active conformation. In contrast, ORG27569, a negative allosteric modulator (NAM) of CB1, also binds to the TM2-TM3-TM4 surface and exerts a NAM effect by impeding the TM2 rearrangement. Our findings fill a gap in the understanding of CB1 allosteric regulation and could guide the rational design of CB1 allosteric modulators. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  7fee.cif.gz 7fee.cif.gz | 224.9 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb7fee.ent.gz pdb7fee.ent.gz | 178.2 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  7fee.json.gz 7fee.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/fe/7fee https://data.pdbj.org/pub/pdb/validation_reports/fe/7fee ftp://data.pdbj.org/pub/pdb/validation_reports/fe/7fee ftp://data.pdbj.org/pub/pdb/validation_reports/fe/7fee | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  7wv9C  5u09S S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data | Similarity search - Function & homology  F&H Search F&H Search |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 63564.348 Da / Num. of mol.: 1 / Mutation: S203K,T210A,E273K,T283V,R340E,N393D Source method: isolated from a genetically manipulated source Details: Cannabinoid receptor 1 inserted with GlgA glycogen synthase Source: (gene. exp.)   Homo sapiens (human), (gene. exp.) Homo sapiens (human), (gene. exp.)    Pyrococcus abyssi GE5 (archaea) Pyrococcus abyssi GE5 (archaea)Gene: CNR1, CNR, PAB2292 / Plasmid: PFASTbac / Cell line (production host): Sf9 / Production host:   Spodoptera frugiperda (fall armyworm) / References: UniProt: P21554, UniProt: Q9V2J8 Spodoptera frugiperda (fall armyworm) / References: UniProt: P21554, UniProt: Q9V2J8 |
|---|
-Non-polymers , 8 types, 34 molecules 
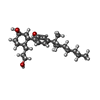
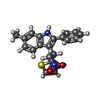












| #2: Chemical |  Cholesterol Cholesterol#3: Chemical | ChemComp-9GF / |  CP 55,940 CP 55,940#4: Chemical | ChemComp-7IC / | #5: Chemical |  Diethylene glycol Diethylene glycol#6: Chemical | ChemComp-GOL /  Glycerol Glycerol#7: Chemical | #8: Chemical | ChemComp-OLA /  Oleic acid Oleic acid#9: Water | ChemComp-HOH / |  Water Water |
|---|
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.72 Å3/Da / Density % sol: 54.78 % |
|---|---|
Crystal grow | Temperature: 293 K / Method: lipidic cubic phase / pH: 6 Details: 25-36% PEG 300, 100Mm MES pH 6.0, 120-190mM Magnesium Sulfate |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SPring-8 SPring-8  / Beamline: BL32XU / Wavelength: 1 Å / Beamline: BL32XU / Wavelength: 1 Å |
| Detector | Type: DECTRIS EIGER X 16M / Detector: PIXEL / Date: Oct 20, 2020 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1 Å / Relative weight: 1 : 1 Å / Relative weight: 1 |
| Reflection | Resolution: 2.7→42.6 Å / Num. obs: 17318 / Redundancy: 32.7 % / Biso Wilson estimate: 86.93 Å2 / CC1/2: 0.987 / Net I/σ(I): 12.6 |
| Reflection shell | Resolution: 2.7→2.79 Å / Num. unique obs: 1694 / CC1/2: 0.733 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 5u09 Resolution: 2.7→42.6 Å / SU ML: 0.52 / Cross valid method: THROUGHOUT / σ(F): 1.33 / Phase error: 38.83 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 192.18 Å2 / Biso mean: 107.2729 Å2 / Biso min: 61.05 Å2 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 2.7→42.6 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Refine-ID: X-RAY DIFFRACTION / Rfactor Rfree error: 0 / Total num. of bins used: 11
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Origin x: 9.5811 Å / Origin y: 14.486 Å / Origin z: 18.5805 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller



 PDBj
PDBj



















