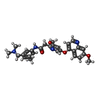
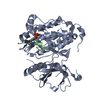
Entry Database : PDB / ID : 6xv9Title Crystal structure of the kinase domain of human c-KIT in complex with a type-II inhibitor Mast/stem cell growth factor receptor Kit,Mast/stem cell growth factor receptor Kit Keywords / / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species Homo sapiens (human)Method / / / / Resolution : 3.38 Å Authors Ogg, D.J. / Howard, T. / McAuley, K. / Hoyt, E.A. / Thomas, M. / Bodnarchuk, M.S. / Lewis, H.J. / Barratt, D. / Deery, M.J. / Bernardes, G.J.L. ...Ogg, D.J. / Howard, T. / McAuley, K. / Hoyt, E.A. / Thomas, M. / Bodnarchuk, M.S. / Lewis, H.J. / Barratt, D. / Deery, M.J. / Bernardes, G.J.L. / Ward, R.A. / Kettle, J.G. / Waring, M.J. Journal : J.Am.Chem.Soc. / Year : 2020Title : Alkynyl Benzoxazines and Dihydroquinazolines as Cysteine Targeting Covalent Warheads and Their Application in Identification of Selective Irreversible Kinase Inhibitors.Authors: McAulay, K. / Hoyt, E.A. / Thomas, M. / Schimpl, M. / Bodnarchuk, M.S. / Lewis, H.J. / Barratt, D. / Bhavsar, D. / Robinson, D.M. / Deery, M.J. / Ogg, D.J. / Bernardes, G.J.L. / Ward, R.A. / ... Authors : McAulay, K. / Hoyt, E.A. / Thomas, M. / Schimpl, M. / Bodnarchuk, M.S. / Lewis, H.J. / Barratt, D. / Bhavsar, D. / Robinson, D.M. / Deery, M.J. / Ogg, D.J. / Bernardes, G.J.L. / Ward, R.A. / Waring, M.J. / Kettle, J.G. History Deposition Jan 21, 2020 Deposition site / Processing site Revision 1.0 May 27, 2020 Provider / Type Revision 1.1 Jun 17, 2020 Group / Category / citation_authorItem _citation.journal_volume / _citation.page_first ... _citation.journal_volume / _citation.page_first / _citation.page_last / _citation_author.name Revision 1.2 May 1, 2024 Group / Database references / Refinement descriptionCategory chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_initial_refinement_model Item / _database_2.pdbx_database_accession
Show all Show less
 Yorodumi
Yorodumi Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords SIGNALING PROTEIN / type II kinase inhibitor /
SIGNALING PROTEIN / type II kinase inhibitor /  structure-based drug design
structure-based drug design Function and homology information
Function and homology information stem cell factor receptor activity / hematopoietic stem cell migration / melanocyte adhesion / positive regulation of pyloric antrum smooth muscle contraction / positive regulation of colon smooth muscle contraction / erythropoietin-mediated signaling pathway / positive regulation of vascular associated smooth muscle cell differentiation / melanocyte migration / positive regulation of dendritic cell cytokine production / Kit signaling pathway / regulation of bile acid metabolic process / positive regulation of small intestine smooth muscle contraction / mast cell differentiation / positive regulation of mast cell proliferation / mast cell chemotaxis / Fc receptor signaling pathway / glycosphingolipid metabolic process / mast cell proliferation / positive regulation of long-term neuronal synaptic plasticity / detection of mechanical stimulus involved in sensory perception of sound / positive regulation of pseudopodium assembly / positive regulation of mast cell cytokine production / immature B cell differentiation / melanocyte differentiation / germ cell migration / lymphoid progenitor cell differentiation / myeloid progenitor cell differentiation / digestive tract development / negative regulation of programmed cell death / embryonic hemopoiesis /
stem cell factor receptor activity / hematopoietic stem cell migration / melanocyte adhesion / positive regulation of pyloric antrum smooth muscle contraction / positive regulation of colon smooth muscle contraction / erythropoietin-mediated signaling pathway / positive regulation of vascular associated smooth muscle cell differentiation / melanocyte migration / positive regulation of dendritic cell cytokine production / Kit signaling pathway / regulation of bile acid metabolic process / positive regulation of small intestine smooth muscle contraction / mast cell differentiation / positive regulation of mast cell proliferation / mast cell chemotaxis / Fc receptor signaling pathway / glycosphingolipid metabolic process / mast cell proliferation / positive regulation of long-term neuronal synaptic plasticity / detection of mechanical stimulus involved in sensory perception of sound / positive regulation of pseudopodium assembly / positive regulation of mast cell cytokine production / immature B cell differentiation / melanocyte differentiation / germ cell migration / lymphoid progenitor cell differentiation / myeloid progenitor cell differentiation / digestive tract development / negative regulation of programmed cell death / embryonic hemopoiesis /  lamellipodium assembly /
lamellipodium assembly /  pigmentation / tongue development / megakaryocyte development / Regulation of KIT signaling / mast cell degranulation / stem cell population maintenance / positive regulation of Notch signaling pathway /
pigmentation / tongue development / megakaryocyte development / Regulation of KIT signaling / mast cell degranulation / stem cell population maintenance / positive regulation of Notch signaling pathway /  cytokine binding / negative regulation of reproductive process / negative regulation of developmental process / spermatid development /
cytokine binding / negative regulation of reproductive process / negative regulation of developmental process / spermatid development /  growth factor binding / somatic stem cell population maintenance /
growth factor binding / somatic stem cell population maintenance /  hemopoiesis / T cell differentiation / ectopic germ cell programmed cell death / hematopoietic progenitor cell differentiation / positive regulation of phospholipase C activity / response to cadmium ion / ovarian follicle development / positive regulation of tyrosine phosphorylation of STAT protein / TFAP2 (AP-2) family regulates transcription of growth factors and their receptors /
hemopoiesis / T cell differentiation / ectopic germ cell programmed cell death / hematopoietic progenitor cell differentiation / positive regulation of phospholipase C activity / response to cadmium ion / ovarian follicle development / positive regulation of tyrosine phosphorylation of STAT protein / TFAP2 (AP-2) family regulates transcription of growth factors and their receptors /  transmembrane receptor protein tyrosine kinase activity /
transmembrane receptor protein tyrosine kinase activity /  SH2 domain binding / cell chemotaxis / B cell differentiation /
SH2 domain binding / cell chemotaxis / B cell differentiation /  erythrocyte differentiation / acrosomal vesicle / Signaling by phosphorylated juxtamembrane, extracellular and kinase domain KIT mutants / epithelial cell proliferation /
erythrocyte differentiation / acrosomal vesicle / Signaling by phosphorylated juxtamembrane, extracellular and kinase domain KIT mutants / epithelial cell proliferation /  stem cell differentiation / positive regulation of receptor signaling pathway via JAK-STAT /
stem cell differentiation / positive regulation of receptor signaling pathway via JAK-STAT /  visual learning / Signaling by SCF-KIT / cytoplasmic side of plasma membrane /
visual learning / Signaling by SCF-KIT / cytoplasmic side of plasma membrane /  receptor protein-tyrosine kinase /
receptor protein-tyrosine kinase /  fibrillar center / cytokine-mediated signaling pathway / male gonad development / Constitutive Signaling by Aberrant PI3K in Cancer / positive regulation of DNA-binding transcription factor activity / cell-cell junction / PIP3 activates AKT signaling / regulation of cell population proliferation / regulation of cell shape / PI5P, PP2A and IER3 Regulate PI3K/AKT Signaling / actin cytoskeleton organization / RAF/MAP kinase cascade /
fibrillar center / cytokine-mediated signaling pathway / male gonad development / Constitutive Signaling by Aberrant PI3K in Cancer / positive regulation of DNA-binding transcription factor activity / cell-cell junction / PIP3 activates AKT signaling / regulation of cell population proliferation / regulation of cell shape / PI5P, PP2A and IER3 Regulate PI3K/AKT Signaling / actin cytoskeleton organization / RAF/MAP kinase cascade /  spermatogenesis /
spermatogenesis /  protein tyrosine kinase activity /
protein tyrosine kinase activity /  protease binding / positive regulation of MAPK cascade / protein autophosphorylation / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction /
protease binding / positive regulation of MAPK cascade / protein autophosphorylation / positive regulation of phosphatidylinositol 3-kinase/protein kinase B signal transduction /  receptor complex / intracellular signal transduction / positive regulation of cell migration /
receptor complex / intracellular signal transduction / positive regulation of cell migration /  inflammatory response
inflammatory response
 Homo sapiens (human)
Homo sapiens (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT /
MOLECULAR REPLACEMENT /  molecular replacement / Resolution: 3.38 Å
molecular replacement / Resolution: 3.38 Å  Authors
Authors Citation
Citation Journal: J.Am.Chem.Soc. / Year: 2020
Journal: J.Am.Chem.Soc. / Year: 2020 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 6xv9.cif.gz
6xv9.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb6xv9.ent.gz
pdb6xv9.ent.gz PDB format
PDB format 6xv9.json.gz
6xv9.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/xv/6xv9
https://data.pdbj.org/pub/pdb/validation_reports/xv/6xv9 ftp://data.pdbj.org/pub/pdb/validation_reports/xv/6xv9
ftp://data.pdbj.org/pub/pdb/validation_reports/xv/6xv9 Links
Links Assembly
Assembly


 Components
Components
 Homo sapiens (human) / Gene: KIT, SCFR / Plasmid: pET28b / Production host:
Homo sapiens (human) / Gene: KIT, SCFR / Plasmid: pET28b / Production host: 
 Escherichia coli (E. coli) / Strain (production host): BL21 Star (DE3)
Escherichia coli (E. coli) / Strain (production host): BL21 Star (DE3) receptor protein-tyrosine kinase
receptor protein-tyrosine kinase X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation
 SYNCHROTRON / Site:
SYNCHROTRON / Site:  Diamond
Diamond  / Beamline: I04 / Wavelength: 0.97949 Å
/ Beamline: I04 / Wavelength: 0.97949 Å : 0.97949 Å / Relative weight: 1
: 0.97949 Å / Relative weight: 1 
 molecular replacement
molecular replacement Processing
Processing :
:  MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller















 PDBj
PDBj