[English] 日本語
 Yorodumi
Yorodumi- PDB-6snd: crystal structure of LN01 Fab in complex with an HIV-1 gp41 peptide -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6snd | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | crystal structure of LN01 Fab in complex with an HIV-1 gp41 peptide | ||||||||||||
 Components Components |
| ||||||||||||
 Keywords Keywords |  IMMUNE SYSTEM / IMMUNE SYSTEM /  gp41 / gp41 /  antibody / antibody /  complex / MPER complex / MPER | ||||||||||||
| Function / homology |  Function and homology information Function and homology information: / Synthesis and processing of ENV and VPU / evasion of host immune response / Alpha-defensins / Dectin-2 family / Binding and entry of HIV virion / positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / positive regulation of establishment of T cell polarity / virus-mediated perturbation of host defense response ...: / Synthesis and processing of ENV and VPU / evasion of host immune response / Alpha-defensins / Dectin-2 family / Binding and entry of HIV virion / positive regulation of plasma membrane raft polarization / positive regulation of receptor clustering / positive regulation of establishment of T cell polarity / virus-mediated perturbation of host defense response / host cell endosome membrane / actin filament organization / Assembly Of The HIV Virion / Budding and maturation of HIV virion / clathrin-dependent endocytosis of virus by host cell /  viral protein processing / symbiont entry into host cell / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane / viral protein processing / symbiont entry into host cell / fusion of virus membrane with host plasma membrane / fusion of virus membrane with host endosome membrane /  viral envelope / virion attachment to host cell / host cell plasma membrane / virion membrane / structural molecule activity / viral envelope / virion attachment to host cell / host cell plasma membrane / virion membrane / structural molecule activity /  membrane / membrane /  plasma membrane plasma membraneSimilarity search - Function | ||||||||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human)   Human immunodeficiency virus 1 Human immunodeficiency virus 1 | ||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3.1 Å MOLECULAR REPLACEMENT / Resolution: 3.1 Å | ||||||||||||
 Authors Authors | Caillat, C. / Pinto, D. / Corti, D. / Fenwick, C. / Pantaleo, G. / Weissenhorn, W. | ||||||||||||
| Funding support |  Switzerland, Switzerland,  France, 3items France, 3items
| ||||||||||||
 Citation Citation |  Journal: Cell Host Microbe / Year: 2019 Journal: Cell Host Microbe / Year: 2019Title: Structural Basis for Broad HIV-1 Neutralization by the MPER-Specific Human Broadly Neutralizing Antibody LN01. Authors: Pinto, D. / Fenwick, C. / Caillat, C. / Silacci, C. / Guseva, S. / Dehez, F. / Chipot, C. / Barbieri, S. / Minola, A. / Jarrossay, D. / Tomaras, G.D. / Shen, X. / Riva, A. / Tarkowski, M. / ...Authors: Pinto, D. / Fenwick, C. / Caillat, C. / Silacci, C. / Guseva, S. / Dehez, F. / Chipot, C. / Barbieri, S. / Minola, A. / Jarrossay, D. / Tomaras, G.D. / Shen, X. / Riva, A. / Tarkowski, M. / Schwartz, O. / Bruel, T. / Dufloo, J. / Seaman, M.S. / Montefiori, D.C. / Lanzavecchia, A. / Corti, D. / Pantaleo, G. / Weissenhorn, W. | ||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6snd.cif.gz 6snd.cif.gz | 408.7 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6snd.ent.gz pdb6snd.ent.gz | 301.7 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6snd.json.gz 6snd.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/sn/6snd https://data.pdbj.org/pub/pdb/validation_reports/sn/6snd ftp://data.pdbj.org/pub/pdb/validation_reports/sn/6snd ftp://data.pdbj.org/pub/pdb/validation_reports/sn/6snd | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| 2 | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments:
NCS ensembles :
|
- Components
Components
-Antibody , 2 types, 4 molecules ALBH
| #1: Antibody | Mass: 23230.734 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Production host: Homo sapiens (human) / Production host:   Homo sapiens (human) Homo sapiens (human)#2: Antibody | Mass: 25520.502 Da / Num. of mol.: 2 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Production host: Homo sapiens (human) / Production host:   Homo sapiens (human) Homo sapiens (human) |
|---|
-Protein/peptide / Sugars , 2 types, 4 molecules CP

| #3: Protein/peptide | Mass: 3292.096 Da / Num. of mol.: 2 / Source method: obtained synthetically / Source: (synth.)    Human immunodeficiency virus 1 / References: UniProt: G3DH64, UniProt: P04578*PLUS Human immunodeficiency virus 1 / References: UniProt: G3DH64, UniProt: P04578*PLUS#4: Sugar |  N-Acetylglucosamine N-Acetylglucosamine |
|---|
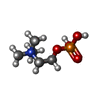
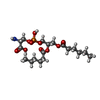
-Non-polymers , 3 types, 3 molecules 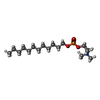


| #5: Chemical | ChemComp-DPV / |
|---|---|
| #6: Chemical | ChemComp-PC /  Phosphocholine Phosphocholine |
| #7: Chemical | ChemComp-PSF /  Phosphatidylserine Phosphatidylserine |
-Details
| Has ligand of interest | Y |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 3.43 Å3/Da / Density % sol: 64.17 % |
|---|---|
Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / Details: 0.1 M Hepes pH 7,5 10% PEG 3350 |
-Data collection
| Diffraction | Mean temperature: 100 K / Serial crystal experiment: N |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID23-1 / Wavelength: 0.98 Å / Beamline: ID23-1 / Wavelength: 0.98 Å |
| Detector | Type: DECTRIS PILATUS 6M-F / Detector: PIXEL / Date: Mar 11, 2018 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.98 Å / Relative weight: 1 : 0.98 Å / Relative weight: 1 |
| Reflection | Resolution: 3.1→48.91 Å / Num. obs: 26213 / % possible obs: 99.8 % / Redundancy: 7.1 % / Biso Wilson estimate: 76.56 Å2 / CC1/2: 0.995 / Rmerge(I) obs: 0.161 / Rpim(I) all: 0.09 / Rrim(I) all: 0.186 / Net I/σ(I): 8.8 |
| Reflection shell | Resolution: 3.1→3.31 Å / Rmerge(I) obs: 0.968 / Mean I/σ(I) obs: 1.9 / Num. unique obs: 4662 / CC1/2: 0.671 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT / Resolution: 3.1→47.17 Å / SU ML: 0.4803 / Cross valid method: FREE R-VALUE / σ(F): 1.33 / Phase error: 27.3254 MOLECULAR REPLACEMENT / Resolution: 3.1→47.17 Å / SU ML: 0.4803 / Cross valid method: FREE R-VALUE / σ(F): 1.33 / Phase error: 27.3254 Stereochemistry target values: GeoStd + Monomer Library + CDL v1.2
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 69.3 Å2 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 3.1→47.17 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller














 PDBj
PDBj















