+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 6fkb | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of N2C/D282C stabilized opsin bound to RS13 | ||||||
 Components Components | Rhodopsin | ||||||
 Keywords Keywords |  MEMBRANE PROTEIN / MEMBRANE PROTEIN /  RHODOPSIN / RHODOPSIN /  G PROTEIN-COUPLED RECEPTORS / G PROTEIN-COUPLED RECEPTORS /  RETINITIS PIGMENTOSA / RETINITIS PIGMENTOSA /  SIGNALING PROTEIN / SIGNALING PROTEIN /  SENSORY TRANSDUCTION / SENSORY TRANSDUCTION /  PHOTORECEPTOR PROTEIN / KINTEGRAL MEMBRANE PROTEIN / VISION / PHOTORECEPTOR PROTEIN / KINTEGRAL MEMBRANE PROTEIN / VISION /  MEMBRANE / MEMBRANE /  RECEPTOR / TRANSDUCER PHOTORECEPTOR / RECEPTOR / TRANSDUCER PHOTORECEPTOR /  SMALL MOLECULE COMPLEX SMALL MOLECULE COMPLEX | ||||||
| Function / homology |  Function and homology information Function and homology information Opsins / VxPx cargo-targeting to cilium / rod bipolar cell differentiation / rod photoreceptor outer segment / sperm head plasma membrane / Opsins / VxPx cargo-targeting to cilium / rod bipolar cell differentiation / rod photoreceptor outer segment / sperm head plasma membrane /  podosome assembly / absorption of visible light / podosome assembly / absorption of visible light /  opsin binding / The canonical retinoid cycle in rods (twilight vision) / : ... opsin binding / The canonical retinoid cycle in rods (twilight vision) / : ... Opsins / VxPx cargo-targeting to cilium / rod bipolar cell differentiation / rod photoreceptor outer segment / sperm head plasma membrane / Opsins / VxPx cargo-targeting to cilium / rod bipolar cell differentiation / rod photoreceptor outer segment / sperm head plasma membrane /  podosome assembly / absorption of visible light / podosome assembly / absorption of visible light /  opsin binding / The canonical retinoid cycle in rods (twilight vision) / : / G protein-coupled photoreceptor activity / photoreceptor inner segment membrane / G protein-coupled opsin signaling pathway / opsin binding / The canonical retinoid cycle in rods (twilight vision) / : / G protein-coupled photoreceptor activity / photoreceptor inner segment membrane / G protein-coupled opsin signaling pathway /  11-cis retinal binding / cellular response to light stimulus / G protein-coupled receptor complex / Inactivation, recovery and regulation of the phototransduction cascade / 11-cis retinal binding / cellular response to light stimulus / G protein-coupled receptor complex / Inactivation, recovery and regulation of the phototransduction cascade /  phototransduction, visible light / phototransduction, visible light /  thermotaxis / Activation of the phototransduction cascade / outer membrane / detection of temperature stimulus involved in thermoception / arrestin family protein binding / thermotaxis / Activation of the phototransduction cascade / outer membrane / detection of temperature stimulus involved in thermoception / arrestin family protein binding /  photoreceptor cell maintenance / photoreceptor outer segment membrane / G alpha (i) signalling events / photoreceptor cell maintenance / photoreceptor outer segment membrane / G alpha (i) signalling events /  phototransduction / response to light stimulus / photoreceptor outer segment / G-protein alpha-subunit binding / sperm midpiece / phototransduction / response to light stimulus / photoreceptor outer segment / G-protein alpha-subunit binding / sperm midpiece /  visual perception / guanyl-nucleotide exchange factor activity / microtubule cytoskeleton organization / photoreceptor disc membrane / cell-cell junction / visual perception / guanyl-nucleotide exchange factor activity / microtubule cytoskeleton organization / photoreceptor disc membrane / cell-cell junction /  gene expression / G protein-coupled receptor signaling pathway / gene expression / G protein-coupled receptor signaling pathway /  Golgi membrane / zinc ion binding / Golgi membrane / zinc ion binding /  membrane / identical protein binding / membrane / identical protein binding /  plasma membrane plasma membraneSimilarity search - Function | ||||||
| Biological species |   Bos taurus (cattle) Bos taurus (cattle) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 3.03 Å MOLECULAR REPLACEMENT / Resolution: 3.03 Å | ||||||
 Authors Authors | Mattle, D. / Standfuss, J. / Dawson, R. | ||||||
| Funding support |  Switzerland, 1items Switzerland, 1items
| ||||||
 Citation Citation |  Journal: Proc. Natl. Acad. Sci. U.S.A. / Year: 2018 Journal: Proc. Natl. Acad. Sci. U.S.A. / Year: 2018Title: Ligand channel in pharmacologically stabilized rhodopsin. Authors: Mattle, D. / Kuhn, B. / Aebi, J. / Bedoucha, M. / Kekilli, D. / Grozinger, N. / Alker, A. / Rudolph, M.G. / Schmid, G. / Schertler, G.F.X. / Hennig, M. / Standfuss, J. / Dawson, R.J.P. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  6fkb.cif.gz 6fkb.cif.gz | 151.8 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb6fkb.ent.gz pdb6fkb.ent.gz | 121.5 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  6fkb.json.gz 6fkb.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/fk/6fkb https://data.pdbj.org/pub/pdb/validation_reports/fk/6fkb ftp://data.pdbj.org/pub/pdb/validation_reports/fk/6fkb ftp://data.pdbj.org/pub/pdb/validation_reports/fk/6fkb | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  6fk6C  6fk7C  6fk8C  6fk9C  6fkaC  6fkcC  6fkdC  4j4qS S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein |  Mass: 37057.574 Da / Num. of mol.: 1 / Fragment: RESIDUES 1-326 / Mutation: YES Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Bos taurus (cattle) / Gene: RHO / Plasmid: PCMV-TET O / Cell line (production host): HEK293S GNTI- / Production host: Bos taurus (cattle) / Gene: RHO / Plasmid: PCMV-TET O / Cell line (production host): HEK293S GNTI- / Production host:   Homo sapiens (human) / References: UniProt: P02699 Homo sapiens (human) / References: UniProt: P02699 |
|---|
-Sugars , 4 types, 10 molecules 






| #3: Sugar |  N-Acetylglucosamine N-Acetylglucosamine#4: Sugar | ChemComp-BMA / |  Mannose Mannose#5: Sugar |  Mannose Mannose#6: Sugar | ChemComp-BOG /  Octyl glucoside Octyl glucoside |
|---|
-Non-polymers , 3 types, 4 molecules 
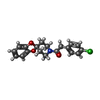



| #2: Chemical | ChemComp-PLM /  Palmitic acid Palmitic acid |
|---|---|
| #7: Chemical | ChemComp-DLH / |
| #8: Water | ChemComp-HOH /  Water Water |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 8.11 Å3/Da |
|---|---|
Crystal grow | Temperature: 277 K / Method: vapor diffusion, sitting drop / Details: see publication |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SLS SLS  / Beamline: X10SA / Wavelength: 1 Å / Beamline: X10SA / Wavelength: 1 Å |
| Detector | Type: DECTRIS PILATUS 6M / Detector: PIXEL / Date: Nov 27, 2015 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 1 Å / Relative weight: 1 : 1 Å / Relative weight: 1 |
| Reflection | Resolution: 3.03→47.55 Å / Num. obs: 24484 / % possible obs: 99.9 % / Redundancy: 11.28 % / Rmerge(I) obs: 0.2624 / Rsym value: 0.2624 / Net I/σ(I): 6.89 |
| Reflection shell | Resolution: 3.03→3.13 Å / Redundancy: 11.81 % / Rmerge(I) obs: 1.0302 / Mean I/σ(I) obs: 0.65 / Rsym value: 1.0302 / % possible all: 100 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 4J4Q Resolution: 3.03→45.895 Å / SU ML: 0.5 / Cross valid method: FREE R-VALUE / σ(F): 0 / Phase error: 33.85 / Stereochemistry target values: ML
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å / Solvent model: FLAT BULK SOLVENT MODEL | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 3.03→45.895 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller













 PDBj
PDBj


















