+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5ovc | ||||||
|---|---|---|---|---|---|---|---|
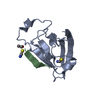
| Title | PDZ domain from rat Shank3 bound to the C terminus of GKAP | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  PROTEIN BINDING / PROTEIN BINDING /  PDZ domain / PDZ domain /  peptide binding / peptide binding /  post-synaptic density / post-synaptic density /  C terminus C terminus | ||||||
| Function / homology |  Function and homology information Function and homology informationresponse to interleukin-17 / regulation of AMPA glutamate receptor clustering / guanylate kinase-associated protein clustering / striatal medium spiny neuron differentiation / synaptic receptor adaptor activity / maintenance of postsynaptic density structure / RET signaling / positive regulation of glutamate receptor signaling pathway /  postsynaptic density assembly / embryonic epithelial tube formation ...response to interleukin-17 / regulation of AMPA glutamate receptor clustering / guanylate kinase-associated protein clustering / striatal medium spiny neuron differentiation / synaptic receptor adaptor activity / maintenance of postsynaptic density structure / RET signaling / positive regulation of glutamate receptor signaling pathway / postsynaptic density assembly / embryonic epithelial tube formation ...response to interleukin-17 / regulation of AMPA glutamate receptor clustering / guanylate kinase-associated protein clustering / striatal medium spiny neuron differentiation / synaptic receptor adaptor activity / maintenance of postsynaptic density structure / RET signaling / positive regulation of glutamate receptor signaling pathway /  postsynaptic density assembly / embryonic epithelial tube formation / postsynaptic specialization / Neurexins and neuroligins / positive regulation of synapse structural plasticity / postsynaptic density assembly / embryonic epithelial tube formation / postsynaptic specialization / Neurexins and neuroligins / positive regulation of synapse structural plasticity /  signaling / negative regulation of actin filament bundle assembly / signaling / negative regulation of actin filament bundle assembly /  vocal learning / negative regulation of cell volume / positive regulation of long-term neuronal synaptic plasticity / structural constituent of postsynaptic density / regulation of grooming behavior / protein localization to synapse / NMDA glutamate receptor clustering / vocalization behavior / regulation of dendritic spine morphogenesis / regulation of behavioral fear response / neuron spine / AMPA glutamate receptor clustering / neural precursor cell proliferation / locomotion / dendritic spine morphogenesis / brain morphogenesis / vocal learning / negative regulation of cell volume / positive regulation of long-term neuronal synaptic plasticity / structural constituent of postsynaptic density / regulation of grooming behavior / protein localization to synapse / NMDA glutamate receptor clustering / vocalization behavior / regulation of dendritic spine morphogenesis / regulation of behavioral fear response / neuron spine / AMPA glutamate receptor clustering / neural precursor cell proliferation / locomotion / dendritic spine morphogenesis / brain morphogenesis /  aggresome assembly / regulation of long-term synaptic potentiation / positive regulation of AMPA receptor activity / long-term synaptic depression / regulation of postsynapse organization / ciliary membrane / exploration behavior / regulation of long-term synaptic depression / aggresome assembly / regulation of long-term synaptic potentiation / positive regulation of AMPA receptor activity / long-term synaptic depression / regulation of postsynapse organization / ciliary membrane / exploration behavior / regulation of long-term synaptic depression /  adult behavior / positive regulation of dendritic spine development / locomotory exploration behavior / adult behavior / positive regulation of dendritic spine development / locomotory exploration behavior /  postsynaptic density, intracellular component / postsynaptic density, intracellular component /  social behavior / social behavior /  associative learning / positive regulation of excitatory postsynaptic potential / neuromuscular process controlling balance / glial cell proliferation / regulation of proteasomal protein catabolic process / associative learning / positive regulation of excitatory postsynaptic potential / neuromuscular process controlling balance / glial cell proliferation / regulation of proteasomal protein catabolic process /  synapse assembly / synapse assembly /  ionotropic glutamate receptor binding / positive regulation of synaptic transmission, glutamatergic / locomotory behavior / ionotropic glutamate receptor binding / positive regulation of synaptic transmission, glutamatergic / locomotory behavior /  learning / long-term synaptic potentiation / positive regulation of long-term synaptic potentiation / G protein-coupled receptor binding / learning / long-term synaptic potentiation / positive regulation of long-term synaptic potentiation / G protein-coupled receptor binding /  regulation of synaptic plasticity / modulation of chemical synaptic transmission / regulation of synaptic plasticity / modulation of chemical synaptic transmission /  memory / memory /  SH3 domain binding / : / SH3 domain binding / : /  MAPK cascade / MAPK cascade /  gene expression / gene expression /  actin binding / actin binding /  scaffold protein binding / scaffold protein binding /  postsynaptic membrane / postsynaptic membrane /  dendritic spine / dendritic spine /  postsynaptic density / learning or memory / molecular adaptor activity / neuron projection / protein domain specific binding / postsynaptic density / learning or memory / molecular adaptor activity / neuron projection / protein domain specific binding /  synapse / glutamatergic synapse / protein-containing complex binding / zinc ion binding / identical protein binding / synapse / glutamatergic synapse / protein-containing complex binding / zinc ion binding / identical protein binding /  plasma membrane / plasma membrane /  cytoplasm cytoplasmSimilarity search - Function | ||||||
| Biological species |   Rattus norvegicus (Norway rat) Rattus norvegicus (Norway rat) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.55 Å MOLECULAR REPLACEMENT / Resolution: 1.55 Å | ||||||
 Authors Authors | Ponna, S.K. / Myllykoski, M. / Boeckers, T.M. / Kursula, P. | ||||||
 Citation Citation |  Journal: J. Neurochem. / Year: 2018 Journal: J. Neurochem. / Year: 2018Title: Structural basis for PDZ domain interactions in the post-synaptic density scaffolding protein Shank3. Authors: Ponna, S.K. / Ruskamo, S. / Myllykoski, M. / Keller, C. / Boeckers, T.M. / Kursula, P. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5ovc.cif.gz 5ovc.cif.gz | 73.3 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5ovc.ent.gz pdb5ovc.ent.gz | 58.6 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5ovc.json.gz 5ovc.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ov/5ovc https://data.pdbj.org/pub/pdb/validation_reports/ov/5ovc ftp://data.pdbj.org/pub/pdb/validation_reports/ov/5ovc ftp://data.pdbj.org/pub/pdb/validation_reports/ov/5ovc | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
| ||||||||
| Components on special symmetry positions |
|
- Components
Components
| #1: Protein | Mass: 10400.004 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Rattus norvegicus (Norway rat) / Gene: Shank3, Prosap2 / Production host: Rattus norvegicus (Norway rat) / Gene: Shank3, Prosap2 / Production host:   Escherichia coli (E. coli) / References: UniProt: Q9JLU4 Escherichia coli (E. coli) / References: UniProt: Q9JLU4 |
|---|---|
| #2: Protein/peptide | Mass: 743.829 Da / Num. of mol.: 1 / Source method: obtained synthetically / Source: (synth.)   Rattus norvegicus (Norway rat) / References: UniProt: P97836*PLUS Rattus norvegicus (Norway rat) / References: UniProt: P97836*PLUS |
| #3: Chemical | ChemComp-CL /  Chloride Chloride |
| #4: Water | ChemComp-HOH /  Water Water |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.44 Å3/Da / Density % sol: 49.54 % |
|---|---|
Crystal grow | Temperature: 293 K / Method: vapor diffusion, sitting drop / Details: 1.6M sodium citrate pH 6.5 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  Diamond Diamond  / Beamline: I04 / Wavelength: 0.9795 Å / Beamline: I04 / Wavelength: 0.9795 Å |
| Detector | Type: DECTRIS PILATUS3 6M / Detector: PIXEL / Date: May 3, 2016 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.9795 Å / Relative weight: 1 : 0.9795 Å / Relative weight: 1 |
| Reflection | Resolution: 1.55→50 Å / Num. obs: 30593 / % possible obs: 99.9 % / Redundancy: 6.7 % / CC1/2: 0.999 / Rrim(I) all: 0.126 / Net I/σ(I): 22.1 |
| Reflection shell | Resolution: 1.55→1.59 Å / Redundancy: 6 % / Num. unique obs: 2244 / CC1/2: 0.132 / Rrim(I) all: 3.628 / % possible all: 99.9 |
- Processing
Processing
| Software |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT / Resolution: 1.55→43.608 Å / SU ML: 0.23 / Cross valid method: FREE R-VALUE / σ(F): 1.32 / Phase error: 24.01 MOLECULAR REPLACEMENT / Resolution: 1.55→43.608 Å / SU ML: 0.23 / Cross valid method: FREE R-VALUE / σ(F): 1.32 / Phase error: 24.01
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.9 Å / VDW probe radii: 1.11 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.55→43.608 Å
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller















 PDBj
PDBj



