[English] 日本語
 Yorodumi
Yorodumi- PDB-5nx9: Crystal structure of Neanderthal Adenylosuccinate Lyase (ADSL) in... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5nx9 | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crystal structure of Neanderthal Adenylosuccinate Lyase (ADSL) in complex with its products AMP and fumarate | |||||||||
 Components Components | Adenylosuccinate lyase | |||||||||
 Keywords Keywords |  LYASE / LYASE /  Adenylosuccinate Lyase / Adenylosuccinate Lyase /  fumarase fumarase | |||||||||
| Function / homology |  Function and homology information Function and homology information adenylosuccinate lyase / adenylosuccinate lyase /  N6-(1,2-dicarboxyethyl)AMP AMP-lyase (fumarate-forming) activity / (S)-2-(5-amino-1-(5-phospho-D-ribosyl)imidazole-4-carboxamido) succinate lyase (fumarate-forming) activity / 'de novo' AMP biosynthetic process / 'de novo' IMP biosynthetic process N6-(1,2-dicarboxyethyl)AMP AMP-lyase (fumarate-forming) activity / (S)-2-(5-amino-1-(5-phospho-D-ribosyl)imidazole-4-carboxamido) succinate lyase (fumarate-forming) activity / 'de novo' AMP biosynthetic process / 'de novo' IMP biosynthetic processSimilarity search - Function | |||||||||
| Biological species |   Homo sapiens neanderthalensis (Neandertal) Homo sapiens neanderthalensis (Neandertal) | |||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 2.3 Å MOLECULAR REPLACEMENT / Resolution: 2.3 Å | |||||||||
 Authors Authors | Van Laer, B. / Kapp, U. / Soler-Lopez, M. / Leonard, G. / Mueller-Dieckmann, C. | |||||||||
 Citation Citation |  Journal: Sci Rep / Year: 2018 Journal: Sci Rep / Year: 2018Title: Molecular comparison of Neanderthal and Modern Human adenylosuccinate lyase. Authors: Bart Van Laer / Ulrike Kapp / Montserrat Soler-Lopez / Kaja Moczulska / Svante Pääbo / Gordon Leonard / Christoph Mueller-Dieckmann /    Abstract: The availability of genomic data from extinct homini such as Neanderthals has caused a revolution in palaeontology allowing the identification of modern human-specific protein substitutions. ...The availability of genomic data from extinct homini such as Neanderthals has caused a revolution in palaeontology allowing the identification of modern human-specific protein substitutions. Currently, little is known as to how these substitutions alter the proteins on a molecular level. Here, we investigate adenylosuccinate lyase, a conserved enzyme involved in purine metabolism for which several substitutions in the modern human protein (hADSL) have been described to affect intelligence and behaviour. During evolution, modern humans acquired a specific substitution (Ala429Val) in ADSL distinguishing it from the ancestral variant present in Neanderthals (nADSL). We show here that despite this conservative substitution being solvent exposed and located distant from the active site, there is a difference in thermal stability, but not enzymology or ligand binding between nADSL and hADSL. Substitutions near residue 429 which do not profoundly affect enzymology were previously reported to cause neurological symptoms in humans. This study also reveals that ADSL undergoes conformational changes during catalysis which, together with the crystal structure of a hitherto undetermined product bound conformation, explains the molecular origin of disease for several modern human ADSL mutants. | |||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5nx9.cif.gz 5nx9.cif.gz | 386.2 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5nx9.ent.gz pdb5nx9.ent.gz | 312.4 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5nx9.json.gz 5nx9.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/nx/5nx9 https://data.pdbj.org/pub/pdb/validation_reports/nx/5nx9 ftp://data.pdbj.org/pub/pdb/validation_reports/nx/5nx9 ftp://data.pdbj.org/pub/pdb/validation_reports/nx/5nx9 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5nx8SC  5nxaC S: Starting model for refinement C: citing same article ( |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Unit cell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Noncrystallographic symmetry (NCS) | NCS domain:
NCS domain segments: Component-ID: 0 / Refine code: 0
NCS ensembles :
|
- Components
Components
-Protein , 1 types, 4 molecules ABCD
| #1: Protein |  Mass: 55216.445 Da / Num. of mol.: 4 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens neanderthalensis (Neandertal) Homo sapiens neanderthalensis (Neandertal)Plasmid: pET14b / Production host:   Escherichia coli BL21(DE3) (bacteria) / Variant (production host): Rosetta 2 Escherichia coli BL21(DE3) (bacteria) / Variant (production host): Rosetta 2References: UniProt: A0A384E0N4*PLUS,  adenylosuccinate lyase adenylosuccinate lyase |
|---|
-Non-polymers , 7 types, 423 molecules 

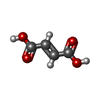










| #2: Chemical | ChemComp-CL /  Chloride Chloride#3: Chemical |  Adenosine monophosphate Adenosine monophosphate#4: Chemical |  Fumaric acid Fumaric acid#5: Chemical | ChemComp-PEG /  Diethylene glycol Diethylene glycol#6: Chemical | #7: Chemical | ChemComp-GOL / |  Glycerol Glycerol#8: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.2 Å3/Da / Density % sol: 58 % |
|---|---|
Crystal grow | Temperature: 277 K / Method: vapor diffusion, sitting drop / pH: 6.5 / Details: 25% PEG1000, 0.1 M MES pH 6.5 |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: MASSIF-3 / Wavelength: 0.967 Å / Beamline: MASSIF-3 / Wavelength: 0.967 Å |
| Detector | Type: DECTRIS PILATUS3 2M / Detector: PIXEL / Date: Nov 20, 2015 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.967 Å / Relative weight: 1 : 0.967 Å / Relative weight: 1 |
| Reflection | Resolution: 2.3→48.38 Å / Num. obs: 86413 / % possible obs: 99.6 % / Redundancy: 3.8 % / Biso Wilson estimate: 38 Å2 / CC1/2: 0.986 / Rrim(I) all: 0.235 / Net I/σ(I): 5.7 |
| Reflection shell | Resolution: 2.3→2.4 Å / Mean I/σ(I) obs: 1.2 / Num. unique obs: 12466 / CC1/2: 0.356 / Rrim(I) all: 1.389 / % possible all: 99.8 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 5NX8 Resolution: 2.3→48.38 Å / Cor.coef. Fo:Fc: 0.952 / Cor.coef. Fo:Fc free: 0.935 / SU B: 9.908 / SU ML: 0.219 / Cross valid method: THROUGHOUT / ESU R: 0.397 / ESU R Free: 0.231 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 40.803 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: 1 / Resolution: 2.3→48.38 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
|
 Movie
Movie Controller
Controller


















 PDBj
PDBj










