[English] 日本語
 Yorodumi
Yorodumi- PDB-5ii5: Crystal structure of red abalone VERL repeat 1 at 1.8 A resolution -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5ii5 | |||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Title | Crystal structure of red abalone VERL repeat 1 at 1.8 A resolution | |||||||||||||||||||||
 Components Components | Maltose-binding periplasmic protein,Vitelline envelope sperm lysin receptor | |||||||||||||||||||||
 Keywords Keywords |  CELL ADHESION / CELL ADHESION /  FERTILIZATION / EGG-SPERM INTERACTION / GAMETE RECOGNITION / FERTILIZATION / EGG-SPERM INTERACTION / GAMETE RECOGNITION /  VITELLINE ENVELOPE / SPERM RECEPTOR VITELLINE ENVELOPE / SPERM RECEPTOR | |||||||||||||||||||||
| Function / homology |  Function and homology information Function and homology information vitelline envelope / sperm-egg recognition / detection of maltose stimulus / vitelline envelope / sperm-egg recognition / detection of maltose stimulus /  maltose binding / maltose transport complex / maltose transport / maltodextrin transmembrane transport / carbohydrate transmembrane transporter activity / ATP-binding cassette (ABC) transporter complex, substrate-binding subunit-containing / carbohydrate transport ... maltose binding / maltose transport complex / maltose transport / maltodextrin transmembrane transport / carbohydrate transmembrane transporter activity / ATP-binding cassette (ABC) transporter complex, substrate-binding subunit-containing / carbohydrate transport ... vitelline envelope / sperm-egg recognition / detection of maltose stimulus / vitelline envelope / sperm-egg recognition / detection of maltose stimulus /  maltose binding / maltose transport complex / maltose transport / maltodextrin transmembrane transport / carbohydrate transmembrane transporter activity / ATP-binding cassette (ABC) transporter complex, substrate-binding subunit-containing / carbohydrate transport / ATP-binding cassette (ABC) transporter complex / cell chemotaxis / outer membrane-bounded periplasmic space / maltose binding / maltose transport complex / maltose transport / maltodextrin transmembrane transport / carbohydrate transmembrane transporter activity / ATP-binding cassette (ABC) transporter complex, substrate-binding subunit-containing / carbohydrate transport / ATP-binding cassette (ABC) transporter complex / cell chemotaxis / outer membrane-bounded periplasmic space /  periplasmic space / DNA damage response / extracellular region / periplasmic space / DNA damage response / extracellular region /  membrane / membrane /  plasma membrane plasma membraneSimilarity search - Function | |||||||||||||||||||||
| Biological species |   Escherichia coli (E. coli) Escherichia coli (E. coli)  Haliotis rufescens (red abalone) Haliotis rufescens (red abalone) | |||||||||||||||||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.8 Å MOLECULAR REPLACEMENT / Resolution: 1.8 Å | |||||||||||||||||||||
 Authors Authors | Sadat Al-Hosseini, H. / Raj, I. / Nishimura, K. / Jovine, L. | |||||||||||||||||||||
| Funding support |  Sweden, 6items Sweden, 6items
| |||||||||||||||||||||
 Citation Citation |  Journal: Cell / Year: 2017 Journal: Cell / Year: 2017Title: Structural Basis of Egg Coat-Sperm Recognition at Fertilization. Authors: Raj, I. / Sadat Al Hosseini, H. / Dioguardi, E. / Nishimura, K. / Han, L. / Villa, A. / de Sanctis, D. / Jovine, L. #1: Journal: Mol. Biol. Evol. / Year: 2011 Title: The molecular basis of sex: linking yeast to human. Authors: Swanson, W.J. / Aagaard, J.E. / Vacquier, V.D. / Monne, M. / Sadat Al Hosseini, H. / Jovine, L. #2: Journal: Proc. Natl. Acad. Sci. U.S.A. / Year: 2006 Title: Rapidly evolving zona pellucida domain proteins are a major component of the vitelline envelope of abalone eggs. Authors: Aagaard, J.E. / Yi, X. / MacCoss, M.J. / Swanson, W.J. #3: Journal: Gene / Year: 2002 Title: Full-length sequence of VERL, the egg vitelline envelope receptor for abalone sperm lysin. Authors: Galindo, B.E. / Moy, G.W. / Swanson, W.J. / Vacquier, V.D. #4: Journal: Proc. Natl. Acad. Sci. U.S.A. / Year: 1997 Title: The abalone egg vitelline envelope receptor for sperm lysin is a giant multivalent molecule. Authors: Swanson, W.J. / Vacquier, V.D. | |||||||||||||||||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5ii5.cif.gz 5ii5.cif.gz | 292.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5ii5.ent.gz pdb5ii5.ent.gz | 239.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5ii5.json.gz 5ii5.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/ii/5ii5 https://data.pdbj.org/pub/pdb/validation_reports/ii/5ii5 ftp://data.pdbj.org/pub/pdb/validation_reports/ii/5ii5 ftp://data.pdbj.org/pub/pdb/validation_reports/ii/5ii5 | HTTPS FTP |
|---|
-Related structure data
| Related structure data |  5ii4C  5ii6C  5ii7C  5ii8C  5ii9C  5iiaC  5iibC  5iicC  5mr2C  5mr3C  3setS  3sexS  4wrnS C: citing same article ( S: Starting model for refinement |
|---|---|
| Similar structure data |
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein | Mass: 54471.543 Da / Num. of mol.: 1 Mutation: N4115Q, N4122T, N4142Y,N4115Q, N4122T, N4142Y,N4115Q, N4122T, N4142Y,N4115Q, N4122T, N4142Y Source method: isolated from a genetically manipulated source Details: THIS PROTEIN IS A CHIMERA. RESIDUES 3667-4034 ARE FROM E. COLI MALTOSE BINDING PROTEIN (MBP), CORRESPOND TO RESIDUES 26-393 OF SWISS-PROT DATABASE ENTRY P0AEX9 AND CONTAIN MUTATIONS A3667T, ...Details: THIS PROTEIN IS A CHIMERA. RESIDUES 3667-4034 ARE FROM E. COLI MALTOSE BINDING PROTEIN (MBP), CORRESPOND TO RESIDUES 26-393 OF SWISS-PROT DATABASE ENTRY P0AEX9 AND CONTAIN MUTATIONS A3667T, D3749A, K3750A, E3839A, N3840A, A3882H, K3886H, K3906A, A3979V, I3984V, E4026A, E4029A, D4030A AND R4034N (CORRESPONDING TO A26T, D108A, K109A, E198A, N199A, A241H, K245H, K265A, A338V, I343V, E385A, E388A, D389A AND R393N IN P0AEX9). RESIDUES 4038-4151 ARE FROM RED ABALONE VITELLINE ENVELOPE SPERM LYSIN RECEPTOR AND CORRESPOND TO RESIDUES 38-151 OF SWISS-PROT DATABASE ENTRY Q8WR62 AND CONTAIN MUTATIONS N4115Q, N4122T AND N4142Y (CORRESPONDING TO N115Q, N122T AND N142Y). Source: (gene. exp.)   Escherichia coli (E. coli), (gene. exp.) Escherichia coli (E. coli), (gene. exp.)   Haliotis rufescens (red abalone) Haliotis rufescens (red abalone)Strain: K12 / Gene: malE, b4034, JW3994, VERL / Plasmid: pHLsec / Cell line (production host): HEK293 / Production host:   Homo sapiens (human) / References: UniProt: P0AEX9, UniProt: Q8WR62 Homo sapiens (human) / References: UniProt: P0AEX9, UniProt: Q8WR62 | ||
|---|---|---|---|
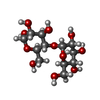
| #2: Polysaccharide | alpha-D-glucopyranose-(1-4)-alpha-D-glucopyranose / alpha-maltose | ||
| #3: Chemical | ChemComp-PGE /  Polyethylene glycol Polyethylene glycol  Mass: 150.173 Da / Num. of mol.: 4 / Mutation: N4115Q, N4122T, N4142Y Mass: 150.173 Da / Num. of mol.: 4 / Mutation: N4115Q, N4122T, N4142YSource method: isolated from a genetically manipulated source Formula: C6H14O4 Details: THIS PROTEIN IS A CHIMERA. RESIDUES 3667-4034 ARE FROM E. COLI MALTOSE BINDING PROTEIN (MBP), CORRESPOND TO RESIDUES 26-393 OF SWISS-PROT DATABASE ENTRY P0AEX9 AND CONTAIN MUTATIONS A3667T, ...Details: THIS PROTEIN IS A CHIMERA. RESIDUES 3667-4034 ARE FROM E. COLI MALTOSE BINDING PROTEIN (MBP), CORRESPOND TO RESIDUES 26-393 OF SWISS-PROT DATABASE ENTRY P0AEX9 AND CONTAIN MUTATIONS A3667T, D3749A, K3750A, E3839A, N3840A, A3882H, K3886H, K3906A, A3979V, I3984V, E4026A, E4029A, D4030A AND R4034N (CORRESPONDING TO A26T, D108A, K109A, E198A, N199A, A241H, K245H, K265A, A338V, I343V, E385A, E388A, D389A AND R393N IN P0AEX9). RESIDUES 4038-4151 ARE FROM RED ABALONE VITELLINE ENVELOPE SPERM LYSIN RECEPTOR AND CORRESPOND TO RESIDUES 38-151 OF SWISS-PROT DATABASE ENTRY Q8WR62 AND CONTAIN MUTATIONS N4115Q, N4122T AND N4142Y (CORRESPONDING TO N115Q, N122T AND N142Y). Source: (gene. exp.)   Escherichia coli (E. coli) / Gene: malE / Plasmid: pHLsec / Cell line (production host): HEK293 / Production host: Escherichia coli (E. coli) / Gene: malE / Plasmid: pHLsec / Cell line (production host): HEK293 / Production host:   Homo sapiens (human) Homo sapiens (human)#4: Water | ChemComp-HOH / |  Water Water |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.99 Å3/Da / Density % sol: 58.9 % |
|---|---|
Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 9.5 / Details: 40% PEG 600, 0.1M CHES |
-Data collection
| Diffraction | Mean temperature: 100 K |
|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: ID29 / Wavelength: 0.97625 Å / Beamline: ID29 / Wavelength: 0.97625 Å |
| Detector | Type: DECTRIS PILATUS 6M-F / Detector: PIXEL / Date: Sep 10, 2014 |
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray |
| Radiation wavelength | Wavelength : 0.97625 Å / Relative weight: 1 : 0.97625 Å / Relative weight: 1 |
| Reflection | Resolution: 1.8→37.837 Å / Num. obs: 61781 / % possible obs: 99 % / Redundancy: 5.9 % / Biso Wilson estimate: 33.37 Å2 / CC1/2: 1 / Rmerge(I) obs: 0.04992 / Net I/σ(I): 17.51 |
| Reflection shell | Resolution: 1.8→1.864 Å / Redundancy: 5.9 % / Rmerge(I) obs: 1.538 / Mean I/σ(I) obs: 1.08 / % possible all: 99 |
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  MOLECULAR REPLACEMENT MOLECULAR REPLACEMENTStarting model: 3SET, 3SEX and 4WRN Resolution: 1.8→37.837 Å / SU ML: 0.24 / Cross valid method: FREE R-VALUE / σ(F): 1.38 / Phase error: 27.38 / Stereochemistry target values: ML
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Shrinkage radii: 0.8 Å / VDW probe radii: 1.1 Å / Solvent model: FLAT BULK SOLVENT MODEL | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 1.8→37.837 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS params. | Method: refined / Refine-ID: X-RAY DIFFRACTION
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement TLS group |
|
 Movie
Movie Controller
Controller

















 PDBj
PDBj