+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 5ccl | ||||||
|---|---|---|---|---|---|---|---|
| Title | Crystal structure of SMYD3 with SAM and oxindole compound | ||||||
 Components Components | Histone-lysine N-methyltransferase SMYD3 | ||||||
 Keywords Keywords | TRANSFERASE/TRANSFERASE Inhibitor / Protein-inhibitor complex /  methyltransferase / methyltransferase /  epigenetics / epigenetics /  drug discovery / TRANSFERASE-TRANSFERASE Inhibitor complex drug discovery / TRANSFERASE-TRANSFERASE Inhibitor complex | ||||||
| Function / homology |  Function and homology information Function and homology informationhistone H3K36 dimethyltransferase activity / histone H4 methyltransferase activity / [histone H3]-lysine4 N-trimethyltransferase / myotube cell development / histone H3K4 trimethyltransferase activity / RNA polymerase II intronic transcription regulatory region sequence-specific DNA binding / RNA polymerase II complex binding / cellular response to dexamethasone stimulus / establishment of protein localization / PKMTs methylate histone lysines ...histone H3K36 dimethyltransferase activity / histone H4 methyltransferase activity / [histone H3]-lysine4 N-trimethyltransferase / myotube cell development / histone H3K4 trimethyltransferase activity / RNA polymerase II intronic transcription regulatory region sequence-specific DNA binding / RNA polymerase II complex binding / cellular response to dexamethasone stimulus / establishment of protein localization / PKMTs methylate histone lysines /  nucleosome assembly / positive regulation of peptidyl-serine phosphorylation / nucleosome assembly / positive regulation of peptidyl-serine phosphorylation /  methylation / RNA polymerase II cis-regulatory region sequence-specific DNA binding / positive regulation of transcription by RNA polymerase II / methylation / RNA polymerase II cis-regulatory region sequence-specific DNA binding / positive regulation of transcription by RNA polymerase II /  nucleoplasm / nucleoplasm /  metal ion binding / metal ion binding /  nucleus / nucleus /  cytosol cytosolSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / Resolution: 1.5 Å SYNCHROTRON / Resolution: 1.5 Å | ||||||
 Authors Authors | Boriack-Sjodin, P.A. | ||||||
 Citation Citation |  Journal: Acs Med.Chem.Lett. / Year: 2016 Journal: Acs Med.Chem.Lett. / Year: 2016Title: Novel Oxindole Sulfonamides and Sulfamides: EPZ031686, the First Orally Bioavailable Small Molecule SMYD3 Inhibitor. Authors: Mitchell, L.H. / Boriack-Sjodin, P.A. / Smith, S. / Thomenius, M. / Rioux, N. / Munchhof, M. / Mills, J.E. / Klaus, C. / Totman, J. / Riera, T.V. / Raimondi, A. / Jacques, S.L. / West, K. / ...Authors: Mitchell, L.H. / Boriack-Sjodin, P.A. / Smith, S. / Thomenius, M. / Rioux, N. / Munchhof, M. / Mills, J.E. / Klaus, C. / Totman, J. / Riera, T.V. / Raimondi, A. / Jacques, S.L. / West, K. / Foley, M. / Waters, N.J. / Kuntz, K.W. / Wigle, T.J. / Scott, M.P. / Copeland, R.A. / Smith, J.J. / Chesworth, R. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  5ccl.cif.gz 5ccl.cif.gz | 220.1 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb5ccl.ent.gz pdb5ccl.ent.gz | 173.1 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  5ccl.json.gz 5ccl.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/cc/5ccl https://data.pdbj.org/pub/pdb/validation_reports/cc/5ccl ftp://data.pdbj.org/pub/pdb/validation_reports/cc/5ccl ftp://data.pdbj.org/pub/pdb/validation_reports/cc/5ccl | HTTPS FTP |
|---|
-Related structure data
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
-Protein , 1 types, 1 molecules A
| #1: Protein | Mass: 49673.633 Da / Num. of mol.: 1 Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: SMYD3, ZMYND1, ZNFN3A1 / Production host: Homo sapiens (human) / Gene: SMYD3, ZMYND1, ZNFN3A1 / Production host:   Escherichia coli (E. coli) Escherichia coli (E. coli)References: UniProt: Q9H7B4,  histone-lysine N-methyltransferase histone-lysine N-methyltransferase |
|---|
-Non-polymers , 5 types, 456 molecules 

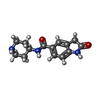






| #2: Chemical | | #3: Chemical | ChemComp-SAM / |  S-Adenosyl methionine S-Adenosyl methionine#4: Chemical | #5: Chemical | ChemComp-EDO / |  Ethylene glycol Ethylene glycol#6: Water | ChemComp-HOH / |  Water Water |
|---|
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.07 Å3/Da / Density % sol: 40.72 % |
|---|---|
Crystal grow | Temperature: 293 K / Method: vapor diffusion, hanging drop / pH: 8 Details: 0.10 M Magnesium formate, 0.1M Tris 8.0, 14% w/v PEG 3350. Crystals of SMYD3-SAM were produced and compound was soaked into the pre-formed crystals |
-Data collection
| Diffraction | Mean temperature: 100 K | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  SSRF SSRF  / Beamline: BL17U / Wavelength: 0.97907 Å / Beamline: BL17U / Wavelength: 0.97907 Å | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: ADSC QUANTUM 315r / Detector: CCD / Date: Dec 15, 2014 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength : 0.97907 Å / Relative weight: 1 : 0.97907 Å / Relative weight: 1 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 1.5→55.55 Å / Num. obs: 62186 / % possible obs: 94.4 % / Redundancy: 5 % / Rmerge(I) obs: 0.069 / Rpim(I) all: 0.033 / Rrim(I) all: 0.077 / Χ2: 1.05 / Net I/av σ(I): 17.437 / Net I/σ(I): 14.6 / Num. measured all: 312049 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1 / Rejects: 0
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Resolution: 1.5→55.55 Å / Cor.coef. Fo:Fc: 0.979 / Cor.coef. Fo:Fc free: 0.961 / SU B: 2.113 / SU ML: 0.037 / Cross valid method: THROUGHOUT / σ(F): 0 / ESU R: 0.078 / ESU R Free: 0.068 / Stereochemistry target values: MAXIMUM LIKELIHOOD Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS U VALUES : REFINED INDIVIDUALLY
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso max: 120.69 Å2 / Biso mean: 22.997 Å2 / Biso min: 8.7 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: final / Resolution: 1.5→55.55 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 1.499→1.538 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller














 PDBj
PDBj





