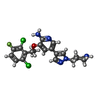
Entry Database : PDB / ID : 4c9wTitle Crystal structure of NUDT1 (MTH1) with R-crizotinib 7,8-DIHYDRO-8-OXOGUANINE TRIPHOSPHATASE Keywords / Function / homology Function Domain/homology Component
/ / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / / Biological species HOMO SAPIENS (human)Method / / / Resolution : 1.65 Å Authors Elkins, J.M. / Salah, E. / Huber, K. / Superti-Furga, G. / Abdul Azeez, K.R. / Raynor, J. / Krojer, T. / von Delft, F. / Bountra, C. / Edwards, A. / Knapp, S. Journal : Nature / Year : 2014Title : Stereospecific Targeting of Mth1 by (S)-Crizotinib as an Anticancer Strategy.Authors: Huber, K.V.M. / Salah, E. / Radic, B. / Gridling, M. / Elkins, J.M. / Stukalov, A. / Jemth, A. / Gokturk, C. / Sanjiv, K. / Stromberg, K. / Pham, T. / Berglund, U.W. / Colinge, J. / Bennett, ... Authors : Huber, K.V.M. / Salah, E. / Radic, B. / Gridling, M. / Elkins, J.M. / Stukalov, A. / Jemth, A. / Gokturk, C. / Sanjiv, K. / Stromberg, K. / Pham, T. / Berglund, U.W. / Colinge, J. / Bennett, K.L. / Loizou, J.I. / Helleday, T. / Knapp, S. / Superti-Furga, G. History Deposition Oct 3, 2013 Deposition site / Processing site Revision 1.0 Apr 2, 2014 Provider / Type Revision 1.1 Apr 16, 2014 Group Revision 1.2 Jan 24, 2018 Group / Category / Item Revision 1.3 Dec 20, 2023 Group Data collection / Database references ... Data collection / Database references / Derived calculations / Other / Refinement description Category chem_comp_atom / chem_comp_bond ... chem_comp_atom / chem_comp_bond / database_2 / pdbx_database_status / pdbx_initial_refinement_model / struct_site Item _database_2.pdbx_DOI / _database_2.pdbx_database_accession ... _database_2.pdbx_DOI / _database_2.pdbx_database_accession / _pdbx_database_status.status_code_sf / _struct_site.pdbx_auth_asym_id / _struct_site.pdbx_auth_comp_id / _struct_site.pdbx_auth_seq_id
Show all Show less
 Open data
Open data Basic information
Basic information Components
Components Keywords
Keywords HYDROLASE /
HYDROLASE /  CRIZOTINIB
CRIZOTINIB Function and homology information
Function and homology information 2-hydroxy-dATP diphosphatase / dATP diphosphatase activity /
2-hydroxy-dATP diphosphatase / dATP diphosphatase activity /  ATP diphosphatase activity / 8-oxo-7,8-dihydrodeoxyguanosine triphosphate pyrophosphatase activity / hydrolase activity, acting on acid anhydrides, in phosphorus-containing anhydrides / 8-oxo-7,8-dihydroguanosine triphosphate pyrophosphatase activity ...2-hydroxy-ATP hydrolase activity / 2-hydroxy-dATP hydrolase activity / N6-methyl-(d)ATP hydrolase activity / O6-methyl-dGTP hydrolase activity /
ATP diphosphatase activity / 8-oxo-7,8-dihydrodeoxyguanosine triphosphate pyrophosphatase activity / hydrolase activity, acting on acid anhydrides, in phosphorus-containing anhydrides / 8-oxo-7,8-dihydroguanosine triphosphate pyrophosphatase activity ...2-hydroxy-ATP hydrolase activity / 2-hydroxy-dATP hydrolase activity / N6-methyl-(d)ATP hydrolase activity / O6-methyl-dGTP hydrolase activity /  2-hydroxy-dATP diphosphatase / dATP diphosphatase activity /
2-hydroxy-dATP diphosphatase / dATP diphosphatase activity /  ATP diphosphatase activity / 8-oxo-7,8-dihydrodeoxyguanosine triphosphate pyrophosphatase activity / hydrolase activity, acting on acid anhydrides, in phosphorus-containing anhydrides / 8-oxo-7,8-dihydroguanosine triphosphate pyrophosphatase activity / DNA protection / Phosphate bond hydrolysis by NUDT proteins / purine nucleoside catabolic process /
ATP diphosphatase activity / 8-oxo-7,8-dihydrodeoxyguanosine triphosphate pyrophosphatase activity / hydrolase activity, acting on acid anhydrides, in phosphorus-containing anhydrides / 8-oxo-7,8-dihydroguanosine triphosphate pyrophosphatase activity / DNA protection / Phosphate bond hydrolysis by NUDT proteins / purine nucleoside catabolic process /  snoRNA binding /
snoRNA binding /  Hydrolases; Acting on acid anhydrides; In phosphorus-containing anhydrides / response to cadmium ion / acrosomal vesicle / male gonad development /
Hydrolases; Acting on acid anhydrides; In phosphorus-containing anhydrides / response to cadmium ion / acrosomal vesicle / male gonad development /  nuclear membrane / response to oxidative stress /
nuclear membrane / response to oxidative stress /  mitochondrial matrix /
mitochondrial matrix /  DNA repair /
DNA repair /  mitochondrion /
mitochondrion /  extracellular space /
extracellular space /  metal ion binding /
metal ion binding /  nucleus /
nucleus /  cytosol /
cytosol /  cytoplasm
cytoplasm
 HOMO SAPIENS (human)
HOMO SAPIENS (human) X-RAY DIFFRACTION /
X-RAY DIFFRACTION /  SYNCHROTRON /
SYNCHROTRON /  MOLECULAR REPLACEMENT / Resolution: 1.65 Å
MOLECULAR REPLACEMENT / Resolution: 1.65 Å  Authors
Authors Citation
Citation Journal: Nature / Year: 2014
Journal: Nature / Year: 2014 Structure visualization
Structure visualization Molmil
Molmil Jmol/JSmol
Jmol/JSmol Downloads & links
Downloads & links Download
Download 4c9w.cif.gz
4c9w.cif.gz PDBx/mmCIF format
PDBx/mmCIF format pdb4c9w.ent.gz
pdb4c9w.ent.gz PDB format
PDB format 4c9w.json.gz
4c9w.json.gz PDBx/mmJSON format
PDBx/mmJSON format Other downloads
Other downloads https://data.pdbj.org/pub/pdb/validation_reports/c9/4c9w
https://data.pdbj.org/pub/pdb/validation_reports/c9/4c9w ftp://data.pdbj.org/pub/pdb/validation_reports/c9/4c9w
ftp://data.pdbj.org/pub/pdb/validation_reports/c9/4c9w
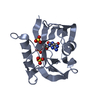
 Links
Links Assembly
Assembly

 Components
Components
 HOMO SAPIENS (human) / Production host:
HOMO SAPIENS (human) / Production host: 
 ESCHERICHIA COLI (E. coli) / Strain (production host): BL21(DE3)
ESCHERICHIA COLI (E. coli) / Strain (production host): BL21(DE3) 8-oxo-dGTP diphosphatase,
8-oxo-dGTP diphosphatase,  2-hydroxy-dATP diphosphatase
2-hydroxy-dATP diphosphatase Chloride
Chloride Crizotinib
Crizotinib Sulfate
Sulfate Water
Water X-RAY DIFFRACTION / Number of used crystals: 1
X-RAY DIFFRACTION / Number of used crystals: 1  Sample preparation
Sample preparation
 SYNCHROTRON / Site:
SYNCHROTRON / Site:  Diamond
Diamond  / Beamline: I04 / Wavelength: 0.9795
/ Beamline: I04 / Wavelength: 0.9795  : 0.9795 Å / Relative weight: 1
: 0.9795 Å / Relative weight: 1  Processing
Processing :
:  MOLECULAR REPLACEMENT
MOLECULAR REPLACEMENT Movie
Movie Controller
Controller













 PDBj
PDBj