[English] 日本語
 Yorodumi
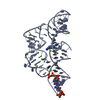
Yorodumi- PDB-3c2i: The Crystal Structure of Methyl-CpG Binding Domain of Human MeCP2... -
+ Open data
Open data
- Basic information
Basic information
| Entry | Database: PDB / ID: 3c2i | ||||||
|---|---|---|---|---|---|---|---|
| Title | The Crystal Structure of Methyl-CpG Binding Domain of Human MeCP2 in Complex with a Methylated DNA Sequence from BDNF | ||||||
 Components Components |
| ||||||
 Keywords Keywords |  Transcription regulator / water mediated recognition / protein-methylated DNA complex / Asx-ST-motif / Disease mutation / DNA-binding / Transcription regulator / water mediated recognition / protein-methylated DNA complex / Asx-ST-motif / Disease mutation / DNA-binding /  Nucleus / Nucleus /  Phosphoprotein / Phosphoprotein /  Repressor Repressor | ||||||
| Function / homology |  Function and homology information Function and homology informationtrans-synaptic signaling by BDNF / regulation of action potential firing threshold / negative regulation of respiratory gaseous exchange / Loss of MECP2 binding ability to 5hmC-DNA / cellular response to isoquinoline alkaloid / positive regulation of anterograde dense core granule transport / positive regulation of retrograde dense core granule transport / positive regulation of branching morphogenesis of a nerve / catecholamine secretion / : ...trans-synaptic signaling by BDNF / regulation of action potential firing threshold / negative regulation of respiratory gaseous exchange / Loss of MECP2 binding ability to 5hmC-DNA / cellular response to isoquinoline alkaloid / positive regulation of anterograde dense core granule transport / positive regulation of retrograde dense core granule transport / positive regulation of branching morphogenesis of a nerve / catecholamine secretion / : / MECP2 regulates transcription of genes involved in GABA signaling / biogenic amine metabolic process / negative regulation of dendrite extension / principal sensory nucleus of trigeminal nerve development / cardiolipin metabolic process / negative regulation of locomotion involved in locomotory behavior / Loss of MECP2 binding ability to 5mC-DNA /  nervous system process involved in regulation of systemic arterial blood pressure / nervous system process involved in regulation of systemic arterial blood pressure /  proprioception / proprioception /  : / negative regulation of primary miRNA processing / negative regulation of smooth muscle cell differentiation / MECP2 regulates transcription of neuronal ligands / Loss of MECP2 binding ability to the NCoR/SMRT complex / negative regulation of dendritic spine development / Transcriptional Regulation by MECP2 / regulation of respiratory gaseous exchange by nervous system process / double-stranded methylated DNA binding / inositol metabolic process / : / negative regulation of primary miRNA processing / negative regulation of smooth muscle cell differentiation / MECP2 regulates transcription of neuronal ligands / Loss of MECP2 binding ability to the NCoR/SMRT complex / negative regulation of dendritic spine development / Transcriptional Regulation by MECP2 / regulation of respiratory gaseous exchange by nervous system process / double-stranded methylated DNA binding / inositol metabolic process /  genomic imprinting / thalamus development / positive regulation of microtubule nucleation / glucocorticoid metabolic process / genomic imprinting / thalamus development / positive regulation of microtubule nucleation / glucocorticoid metabolic process /  ventricular system development / cellular response to potassium ion / unmethylated CpG binding / phosphatidylcholine metabolic process / positive regulation of synaptic plasticity / respiratory gaseous exchange by respiratory system / oligodendrocyte development / olfactory bulb development / striatum development / positive regulation of dendrite extension / response to other organism / siRNA binding / neuron maturation / methyl-CpG binding / MECP2 regulates transcription factors / Loss of phosphorylation of MECP2 at T308 / response to ionizing radiation / spinal cord development / positive regulation of dendritic spine development / regulation of synapse organization / lung alveolus development / glutamine metabolic process / ventricular system development / cellular response to potassium ion / unmethylated CpG binding / phosphatidylcholine metabolic process / positive regulation of synaptic plasticity / respiratory gaseous exchange by respiratory system / oligodendrocyte development / olfactory bulb development / striatum development / positive regulation of dendrite extension / response to other organism / siRNA binding / neuron maturation / methyl-CpG binding / MECP2 regulates transcription factors / Loss of phosphorylation of MECP2 at T308 / response to ionizing radiation / spinal cord development / positive regulation of dendritic spine development / regulation of synapse organization / lung alveolus development / glutamine metabolic process /  startle response / dendrite development / startle response / dendrite development /  social behavior / negative regulation of blood vessel endothelial cell migration / Regulation of MECP2 expression and activity / negative regulation of astrocyte differentiation / behavioral fear response / glial cell proliferation / Nuclear events stimulated by ALK signaling in cancer / social behavior / negative regulation of blood vessel endothelial cell migration / Regulation of MECP2 expression and activity / negative regulation of astrocyte differentiation / behavioral fear response / glial cell proliferation / Nuclear events stimulated by ALK signaling in cancer /  heterochromatin / heterochromatin /  long-term memory / heterochromatin formation / four-way junction DNA binding / MECP2 regulates neuronal receptors and channels / positive regulation of glial cell proliferation / long-term memory / heterochromatin formation / four-way junction DNA binding / MECP2 regulates neuronal receptors and channels / positive regulation of glial cell proliferation /  Notch signaling pathway / sensory perception of pain / Notch signaling pathway / sensory perception of pain /  synapse assembly / histone reader activity / synapse assembly / histone reader activity /  excitatory postsynaptic potential / negative regulation of angiogenesis / adult locomotory behavior / cerebellum development / post-embryonic development / response to cocaine / molecular condensate scaffold activity / promoter-specific chromatin binding / long-term synaptic potentiation / hippocampus development / response to lead ion / excitatory postsynaptic potential / negative regulation of angiogenesis / adult locomotory behavior / cerebellum development / post-embryonic development / response to cocaine / molecular condensate scaffold activity / promoter-specific chromatin binding / long-term synaptic potentiation / hippocampus development / response to lead ion /  visual learning / visual learning /  protein localization / chromatin DNA binding / cerebral cortex development / protein localization / chromatin DNA binding / cerebral cortex development /  histone deacetylase binding / transcription corepressor activity / response to estradiol / histone deacetylase binding / transcription corepressor activity / response to estradiol /  heart development / heart development /  gene expression / postsynapse / negative regulation of neuron apoptotic process / gene expression / postsynapse / negative regulation of neuron apoptotic process /  nucleic acid binding / molecular adaptor activity / response to hypoxia / protein domain specific binding nucleic acid binding / molecular adaptor activity / response to hypoxia / protein domain specific bindingSimilarity search - Function | ||||||
| Biological species |   Homo sapiens (human) Homo sapiens (human) | ||||||
| Method |  X-RAY DIFFRACTION / X-RAY DIFFRACTION /  SYNCHROTRON / SYNCHROTRON /  SAD / Resolution: 2.5 Å SAD / Resolution: 2.5 Å | ||||||
 Authors Authors | Ho, K.L. / McNae, I.W. / Schmiedeberg, L. / Klose, R.J. / Bird, A.P. / Walkinshaw, M.D. | ||||||
 Citation Citation |  Journal: Mol.Cell / Year: 2008 Journal: Mol.Cell / Year: 2008Title: MeCP2 binding to DNA depends upon hydration at methyl-CpG Authors: Ho, K.L. / McNae, I.W. / Schmiedeberg, L. / Klose, R.J. / Bird, A.P. / Walkinshaw, M.D. | ||||||
| History |
|
- Structure visualization
Structure visualization
| Structure viewer | Molecule:  Molmil Molmil Jmol/JSmol Jmol/JSmol |
|---|
- Downloads & links
Downloads & links
- Download
Download
| PDBx/mmCIF format |  3c2i.cif.gz 3c2i.cif.gz | 85.6 KB | Display |  PDBx/mmCIF format PDBx/mmCIF format |
|---|---|---|---|---|
| PDB format |  pdb3c2i.ent.gz pdb3c2i.ent.gz | 66.8 KB | Display |  PDB format PDB format |
| PDBx/mmJSON format |  3c2i.json.gz 3c2i.json.gz | Tree view |  PDBx/mmJSON format PDBx/mmJSON format | |
| Others |  Other downloads Other downloads |
-Validation report
| Arichive directory |  https://data.pdbj.org/pub/pdb/validation_reports/c2/3c2i https://data.pdbj.org/pub/pdb/validation_reports/c2/3c2i ftp://data.pdbj.org/pub/pdb/validation_reports/c2/3c2i ftp://data.pdbj.org/pub/pdb/validation_reports/c2/3c2i | HTTPS FTP |
|---|
-Related structure data
| Similar structure data |
|---|
- Links
Links
- Assembly
Assembly
| Deposited unit | 
| ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 |
| ||||||||
| Unit cell |
|
- Components
Components
| #1: Protein |  MECP2 / MeCP-2 protein / MeCP2 MECP2 / MeCP-2 protein / MeCP2Mass: 11281.381 Da / Num. of mol.: 1 / Fragment: UNP residues 77-167, Human MeCP2 MBD domain / Mutation: A140(MSE) Source method: isolated from a genetically manipulated source Source: (gene. exp.)   Homo sapiens (human) / Gene: MECP2 / Plasmid: pET30b / Production host: Homo sapiens (human) / Gene: MECP2 / Plasmid: pET30b / Production host:   Escherichia coli (E. coli) / Strain (production host): BL21(DE3)pLys / References: UniProt: P51608 Escherichia coli (E. coli) / Strain (production host): BL21(DE3)pLys / References: UniProt: P51608 |
|---|---|
| #2: DNA chain | Mass: 6138.003 Da / Num. of mol.: 1 / Source method: obtained synthetically / Details: synthetic oligonucleotide |
| #3: DNA chain | Mass: 6156.032 Da / Num. of mol.: 1 / Source method: obtained synthetically / Details: synthetic oligonucleotide |
| #4: Water | ChemComp-HOH /  Water Water |
-Experimental details
-Experiment
| Experiment | Method:  X-RAY DIFFRACTION / Number of used crystals: 1 X-RAY DIFFRACTION / Number of used crystals: 1 |
|---|
- Sample preparation
Sample preparation
| Crystal | Density Matthews: 2.21 Å3/Da / Density % sol: 44.33 % | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
Crystal grow | Temperature: 290 K / Method: vapor diffusion, hanging drop / pH: 6.5 Details: 26% PEG2000, 0.2M Ammonium acetate, 0.01M Mg acetate, 0.05M Na cacodylate pH6.5, 0.002M DTT, VAPOR DIFFUSION, HANGING DROP, temperature 290K | ||||||||||||||||||||||||||||||||||||||||||||
| Components of the solutions |
|
-Data collection
| Diffraction | Mean temperature: 100 K | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Diffraction source | Source:  SYNCHROTRON / Site: SYNCHROTRON / Site:  ESRF ESRF  / Beamline: BM14 / Wavelength: 0.978 Å / Beamline: BM14 / Wavelength: 0.978 Å | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Detector | Type: MARMOSAIC 225 mm CCD / Detector: CCD / Date: Aug 7, 2006 / Details: mirrors | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation | Monochromator: silicon / Protocol: SINGLE WAVELENGTH / Monochromatic (M) / Laue (L): M / Scattering type: x-ray | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Radiation wavelength | Wavelength : 0.978 Å / Relative weight: 1 : 0.978 Å / Relative weight: 1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection | Resolution: 2.5→48.795 Å / Num. all: 7122 / Num. obs: 7122 / % possible obs: 98.5 % / Observed criterion σ(F): 0 / Observed criterion σ(I): 0 / Redundancy: 7.1 % / Biso Wilson estimate: 84.3 Å2 / Rmerge(I) obs: 0.075 / Rsym value: 0.075 / Net I/σ(I): 3.1 | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Reflection shell | Diffraction-ID: 1
|
-Phasing
Phasing | Method:  SAD SAD | |||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phasing MAD | D res high: 2.5 Å / D res low: 1000 Å / FOM : 0.25 / Reflection: 7115 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Phasing MAD set site |
| |||||||||||||||||||||||||||||||||||||||||||||||||
| Phasing MAD shell |
| |||||||||||||||||||||||||||||||||||||||||||||||||
| Phasing dm | FOM : 0.53 / FOM acentric: 0.53 / FOM centric: 0.55 / Reflection: 7115 / Reflection acentric: 6648 / Reflection centric: 467 | |||||||||||||||||||||||||||||||||||||||||||||||||
| Phasing dm shell |
|
- Processing
Processing
| Software |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Refinement | Method to determine structure : :  SAD / Resolution: 2.5→23.48 Å / Cor.coef. Fo:Fc: 0.955 / Cor.coef. Fo:Fc free: 0.924 / SU B: 24.894 / SU ML: 0.266 / Cross valid method: THROUGHOUT / σ(F): 0 / σ(I): 0 / ESU R: 0.651 / ESU R Free: 0.325 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS SAD / Resolution: 2.5→23.48 Å / Cor.coef. Fo:Fc: 0.955 / Cor.coef. Fo:Fc free: 0.924 / SU B: 24.894 / SU ML: 0.266 / Cross valid method: THROUGHOUT / σ(F): 0 / σ(I): 0 / ESU R: 0.651 / ESU R Free: 0.325 / Stereochemistry target values: MAXIMUM LIKELIHOOD / Details: HYDROGENS HAVE BEEN ADDED IN THE RIDING POSITIONS
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Solvent computation | Ion probe radii: 0.8 Å / Shrinkage radii: 0.8 Å / VDW probe radii: 1.2 Å / Solvent model: MASK | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Displacement parameters | Biso mean: 59.076 Å2
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refinement step | Cycle: LAST / Resolution: 2.5→23.48 Å
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Refine LS restraints |
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| LS refinement shell | Resolution: 2.5→2.565 Å / Total num. of bins used: 20
|
 Movie
Movie Controller
Controller











 PDBj
PDBj














































